The Utah-based company Merit Medical Systems, Inc. recently announced they’ve secured 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the Elation Pulmonary Dilation Balloon. The product is meant for endoscopically dilating strictures of the trachea or bronchi.
According to company literature, the Elation provides “precise, controlled, three stage dilation and crystal clear visualization during balloon endoscopy.”
The clear visualization is delivered by the unique design of the balloon. In addition, the sizing of the balloon is trumpeted as “accurate and repeatable.
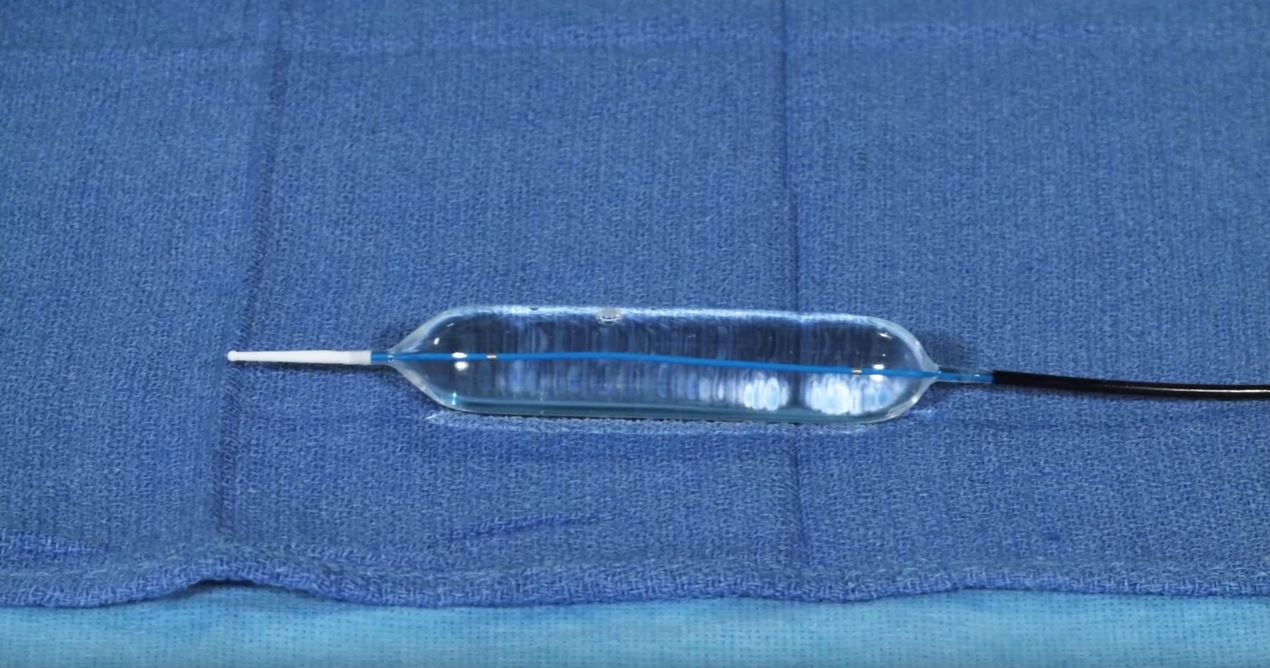
(Image credit: Merit Medical promotional video)
The Elation completely deflates quickly, allowing physicians to expediently withdraw it from the endoscope. In a series of comparison tests conducted by Merit Medical, the Elation consistently deflated in six seconds, as much as twice as fast as competing balloon dilator products.
The Elation has been designed to work with Endotek’s BIG60 Inflation Device.




