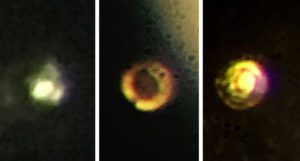
Transparent molecular hydrogen (left) at about 200 GPa became black molecular hydrogen (center) and finally reflective atomic metallic hydrogen at 495 GPa (right). [Image courtesy of Isaac Silvera/Harvard]
If the claims pan out, they could open up a host of possibilities, including in the medical device field. That’s because metallic hydrogen theoretically should be superconductive at room temperature. MRIs, for example, would no longer need supercooled magnets.
Natural sciences professor Isaac Silvera, who worked with postdoctoral fellow Ranga Dias on the metallic hydrogen project, imagines electric grids across the country able to transmit without dissipation. NASA has supported some of the research because the amount of energy stored in the metallic hydrogen could make it the ultimate rocket fuel, enabling easy exploration of the Solar System’s outer planets.
Even better, the theories around metallic hydrogen suggest that it should be meta-stable. “That means if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure but remain diamonds when that pressure and heat are removed,” Silvera said in a Harvard news release.
“This is the Holy Grail of high-pressure physics,” Silvera said. “It’s the first-ever sample of metallic hydrogen on Earth, so when you’re looking at it, you’re looking at something that’s never existed before.”
Some of Silvera and Dias’s peers so far are skeptical of the results, which were published Jan. 26 in the journal Science. They pointed out that Silvera and Dias have yet to reproduce the results.
“It’s—how should I put it?—the product of Ike’s imagination from the title to the end,” Eugene Gregoryanz, a physicist at the University of Edinburgh in Scotland, told The New York Times.
Silvera responded: “If we did it again, we’d get the same result, I’m certain.” He insists that more tests are coming.
Silvera and Dias turned the molecular hydrogen into metallic hydrogen by crushing it in between two diamonds mounted opposite each other in a device known as a diamond anvil cell. But these weren’t any ordinary rocks, according to the researchers. The carefully polished synthetic diamonds first got a 5-micron shave via a reactive ion etching process, removing small defects left from the diamond powder polishing process. Silvera and Dias then coated the diamonds with a thin layer of alumina to prevent the hydrogen from diffusing into the crystal structure of the diamonds and embrittling it.
The super diamonds produced a result that researchers have been seeking for decades, according to Silvera.
“It was really exciting,” Silvera said. “Ranga was running the experiment, and we thought we might get there, but when he called me and said, ‘The sample is shining,’ I went running down there, and it was metallic hydrogen.”
“I immediately said we have to make the measurements to confirm it, so we rearranged the lab … and that’s what we did.”
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]