Researchers from Lomonosov Moscow State University, in cooperation with colleagues from the University of Washington, have developed a method for designing therapeutic transducers that utilize nonlinear focused ultrasound for noninvasive ablation of tumors at deep tissue sites inside the body. Results of this study were recently published in the journal IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control.
An international research team that includes physicists from Moscow State University (MSU) is studying the effect of high intensity focused ultrasound (HIFU) waves to destroy tumors inside the human body noninvasively, without surgical intervention. This therapeutic approach has been pursued for about 25 years; more recently, laboratory experiments are being translated to the clinic.
During the last decade, HIFU researchers explored thermally ablating tumor tissues in prostate, kidney, liver, bones and the brain. Research continues on the potential for treating many other organs.
(Credit: Getty Images)
Recently, researchers began studying high-power therapeutic systems capable of generating nonlinear waves at the focus of HIFU beams in tissue. The shape of such waves is distorted by nonlinear propagation effects and can even contain high-amplitude shock fronts. Ultrasound waves with shocks not only heat tissue much faster than harmonic waves, but can also induce a broader range of possible bioeffects in tissue. Many ideas for exploiting these capabilities have been proposed; however, it was not known what transducer parameters would be needed to deliver nonlinear HIFU treatments that utilize shocks with specified amplitude.
Vera Khokhlova, D.Sc., who supervised these studies at the Department of Acoustics of MSU, said, “Not long ago—about two years—our group was asked about the type of transducers required to form a shock front of desired amplitude at the focus. This nonlinear inverse problem involves consideration of many physical parameters and no solution was known. It was necessary to understand the structure of nonlinear ultrasound fields in biological tissue, to choose adequate mathematical models for governing such fields, and to develop corresponding computational algorithms to work with these models.”
Although the problem was challenging, the group found a solution, demonstrating that the primary transducer parameter is the angle of convergence that determines how strongly ultrasound beam is focused. It was proved that the larger the convergence angle, the higher the shock amplitude that can be achieved at the focus. Although this result may be intuitively obvious, no quantitative assessment of the problem had been performed. In the study, the problem was solved quantitatively using numerical methods and the solution was validated experimentally. For example, the authors have demonstrated that a shock front of 100 MPa amplitude at the focus requires a transducer focusing angle of 60 degrees. If a lower shock amplitude is required, say 35 MPa, then the angle of convergence should be 20 degrees.
These results are critical for a new HIFU technology termed “boiling histotripsy” that is under development by the University of Washington and Moscow State University teams. The term “histotripsy” refers to mechanical disintegration of tissue. So far, the clinical use of HIFU for tumor ablation has been limited to the heating of tissue to high temperatures by ultrasound waves. However, despite recent clinical success of HIFU, the thermal nature of treatments limits its applications in certain clinical situations. The effects of heat diffusion and perfusion lead to uncertainty of ablated tissue volumes and difficulties in treating tissue close to vessels, bones, and other critical structures. Moreover, the treatments are not visible using conventional ultrasound imaging methods; therefore, expensive MR imaging is necessary for monitoring such surgeries in real time.
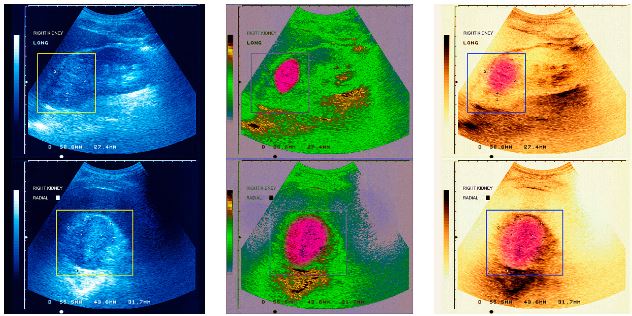
Histotripsy methods of mechanical tissue ablation using shock waves can overcome these challenges. Two methods are under development in parallel by scientists from the University of Michigan (UM) and from the University of Washington (UW, Seattle) in cooperation with their colleagues from Moscow State University (MSU, Moscow). Scientists from UM successfully fractionated tissue in the focal region of the ultrasound beam by generating a cavitation cloud using microsecond-long ultrasound pulses of very high amplitude. Scientists from Seattle and Moscow have found a different solution to the same problem. They use millisecond-long pulses of lower amplitude. Due to nonlinear propagation effects, shock fronts form in a small region (about 1 mm length and 0.1 mm diameter) close to the beam focus. These shocks heat tissue to high temperatures within each pulse, resulting in explosive localized boiling and formation of a millimeter-sized vapor bubble at the focus. This bubble grows so fast that it blocks the path of the ultrasound beam before the end of each pulse.
In the other words, the tail of the pulse consisting of several hundreds of sequential shocks impinges on a vapor bubble rather than propagating in tissue. In this situation, interaction of shocks with the interface between tissue and vapor results in a known physical phenomenon described as an acoustic fountain or ultrasonic atomization. As a result, tissue is disintegrated into micron-sized particles jetting into the cavity and forming a volume of liquefied tissue at the focus.
Vera Khokhlova thinks that these two methods of mechanical tissue fractionation open novel possibilities in ultrasound surgery. Histotripsy treatments are free from uncertainties such as thermal diffusion. Importantly, both cavitation clouds and vapor bubbles can be readily imaged in real time using conventional ultrasonography methods.
