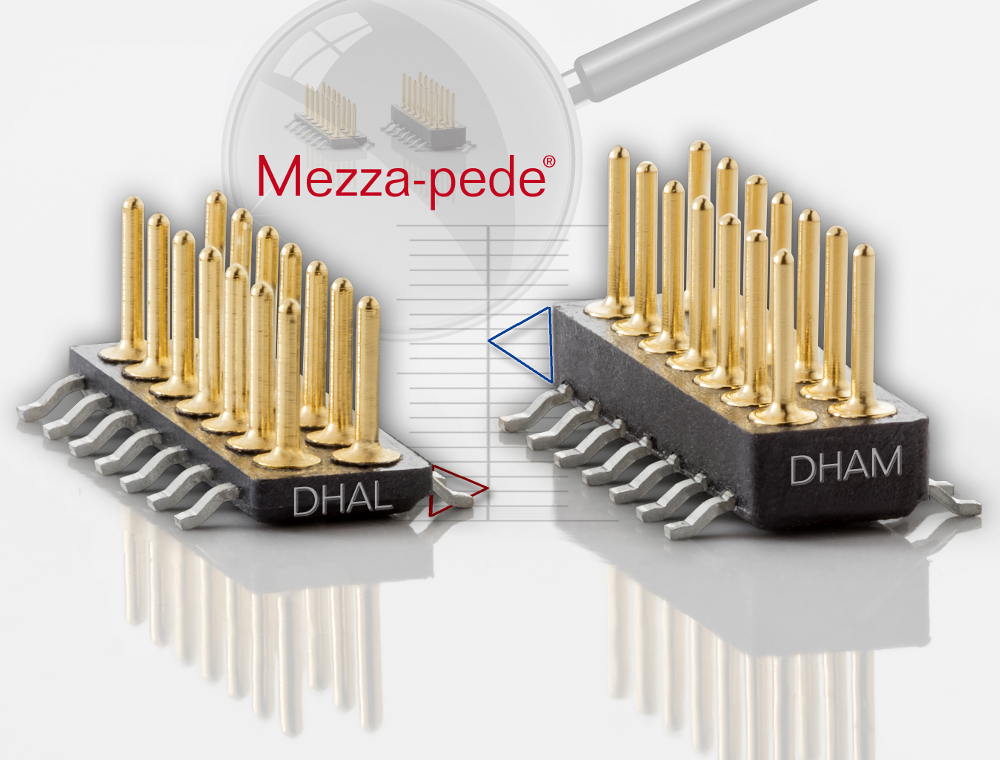
(Credit: Advanced Interconnections Corp.)
Advanced Interconnections Corp. has extended its line of Mezza-pede® SMT Connectors with a new lower profile header (model DHAL) which reduces board stack height by 15%. The innovative design features an ultrathin insulator, at only 0.025 inch (0.63mm) – half the thickness of the original header design. When paired with mating socket model DHS, a z-axis stack height of 0.132 in. (3.4mm) is achievable. This newest Mezza-pede® connector facilitates a reduction in package height and a shorter signal path in applications ranging from tunable laser power connectors in optical transceivers to hand-held medical electronic devices.
Mezza-pede® SMT Connectors are ideal for 1.0mm pitch board-to-board and cable-to-board interconnections in high density electronic designs, where space is at a premium. The new model DHAL is currently available with 14 positions and features Advanced’s proprietary SMT lead frame and pin design, combining high reliability screw-machined terminals with a unique over-molded lead frame in a precision-molded LCP insulator.
Typical applications include telecommunications, military, medical, and anywhere a high reliability surface mount connector is needed in a high density package. Mezza-pede® SMT Connectors with 30 microinches (µin) gold plating pass the 20-day mixed flowing gas (MFG) test, often required by the world’s most demanding telecommunications applications. The Mezza-pede connector line, including the new model DHAL, is RoHS compliant, usable at operating temperatures from -55 to +125°C, and can handle as much as 1.1 A current at +80°C.
Advanced Interconnections Corporation designs and manufactures electronic interconnect solutions, specializing in customized PCB connector and IC socketing and adapter solutions for high reliability applications, with world headquarters located in Rhode Island (USA). For more information, contact Advanced Interconnections’ customer service department at: 5 Energy Way, West Warwick, RI 02893 USA. Tel: 1 (401) 823-5200.




