We are experiencing a tremendous upsurge in the medications delivered transdermally. This drug delivery system provides several benefits over traditional methods, including systemic delivery of the medication to the patient. In particular, microneedle patches and other products have great potential for vaccine delivery. This article will explore the material components that go into the manufacture of microneedle-based delivery systems, as they are crucial for ensuring the highest quality and performance.
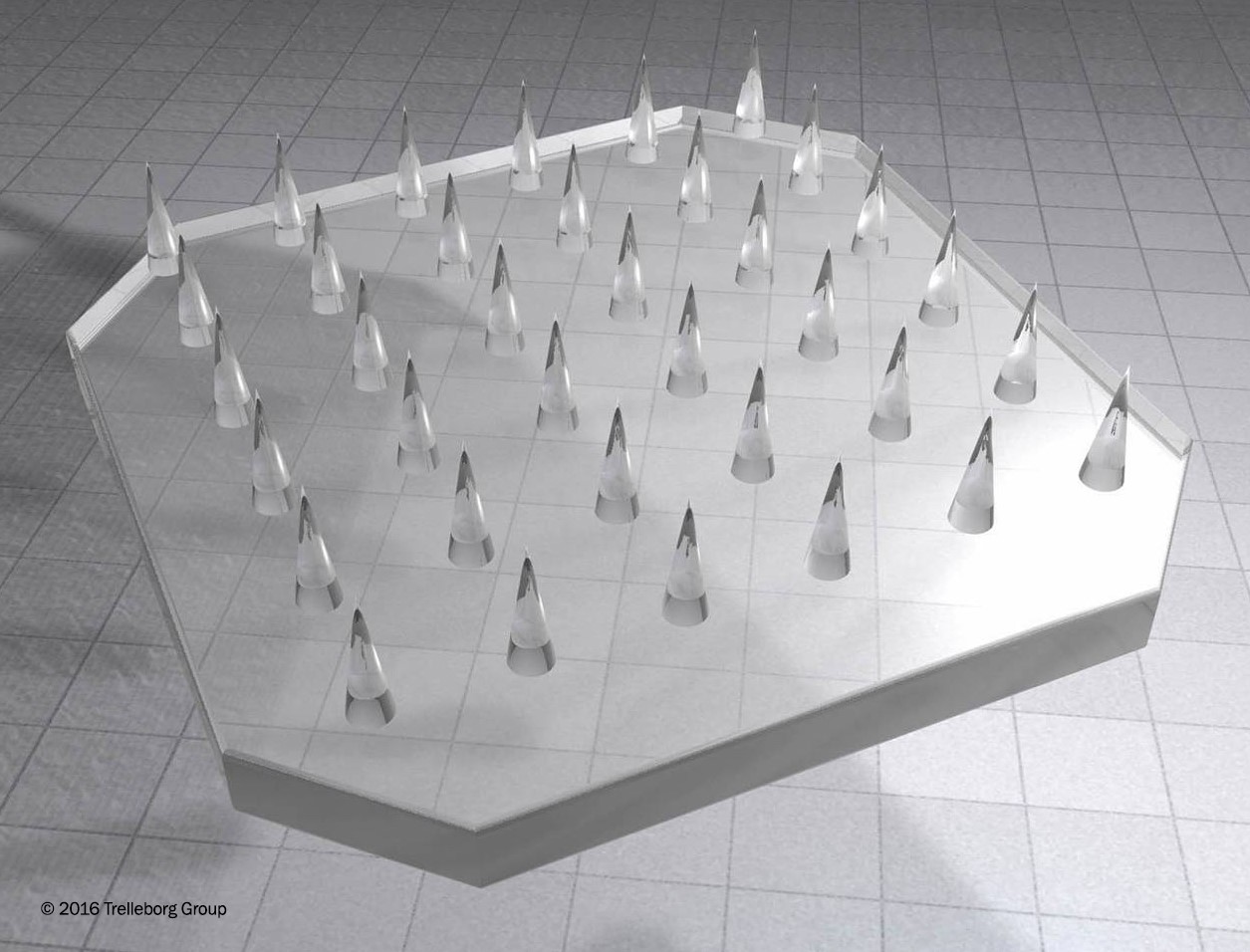
A microneedle array. Image courtesy of Trelleborg Sealing Solutions tss.trelleborg.com
Optimum Material for Optimum Results
There are four types of microneedles currently on the market:
1. Hollow microneedle: requires a liquid drug formulation to be infused through the bores.
2. Solid microneedle: punctures holes in the skin where a patch can then be applied.
3. Dissolving microneedle: coated with the drug.
4. Polymer microneedles: made from special polymers offering dissolving, non-dissolving or hydrogel-forming options.
In each case, these microneedle patch types offer an excellent delivery route to enhance the vaccination’s effectiveness. This is due to the fact that microneedles possess the ability to target the rich network of immunologic antigen-presenting cells in the dermis and epidermis layers under the skin. Many new studies show that microneedle use for vaccination delivery reveals either comparable or greater immunogenicity, a stronger level of stability, and more advantageous dose sparing as compared to the traditional intramuscular routes.
Advanced technologies are coming into play in enhancing microneedle components during the design process. For example, product developers and research institutes are looking at the use of liquid silicone rubber (LSR) technology and two-component injection technology to enhance the performance of their transdermal delivery systems.
Silicone – and LSR in particular – is becoming an increasingly attractive choice of polymer due to a number of advantages. Silicone is well regarded for its favorable haptic properties and proven to generally not cause skin irritation. In addition, silicone provides biocompatibility and compliance with relevant industry regulations. Most importantly, LSR offers fast, essentially unlimited processing possibilities for the most complex high-precision technical components in large volumes.
LSR technology is particularly effective when custom solutions are needed, due to its adaptability. It is also well suited when multiple materials or layers of materials need to be combined into a composite structure. For surface enhancements and surface texturing, LSR provides the intended absorption of medicine through the skin. Lastly, LSR works extremely well when the most complex, thin, and/or tiny features are needed, such as a protective element or carrier as part of a microneedle patch. It provides the highest component precision and consistency of quality for the best possible support to human health.
Advancements in drug delivery systems will be the result of access to newly developed materials, emerging technological delivery methods and advances in manufacturing capabilities. This is precisely what LSR injection technology is offering. LSR technology can deliver smaller, more robust and stronger polymers to provide more stability, wear and usage. The more demanding requirements of pharmaceutical companies and device manufacturers have led to a more concentrated effort to deliver breakthroughs in LSR technology and microfabrication. This necessitates the incorporation of the smallest of parts, down to micro and already nano-gram weights.
Microneedles and Microfabrication Manufacturing
Microneedles consist of a plurality of micro-projections, generally ranging from 25–2000 μm in height, of different shapes, which are attached to a base support. Microfabrication manufacturing technology can help in delivering innovative microneedle designs. There are numerous configurations that can compose a microneedle patch. The flexibility of LSR can assist in achieving those configurations regardless of complexity.
The first microneedle devices were fabricated from silicone but many other materials have also been used in its fabrication like stainless steel, dextrin, glass, ceramic, maltose, galactose, and various polymers.
Recently, the manufacturing of microneedles has encompassed conventional microelectronic fabrication technologies, including chemical isotropic etching, injection molding, reactive ion etching, surface/bulk micromachining, polysilicon micro-molding, lithography-electroforming-replication, and laser drilling. Microneedles have been fabricated with a wide range of designs (different sizes and shapes) and different types (solid, hollow, sharp, or flat).
Forecast
The opportunities to enhance transdermal delivery system design is indeed an exciting one. Device manufacturers are investing in R&D and design strategies to support the transdermal drug delivery industry, valued at $13.5 billion in 2013 and expected to reach $21.7 billion by 2018 according to the Micro-market Monitor.
Microneedles are not limited to any specific class of drugs. According to “The Microneedles for Transdermal and Intradermal Drug Delivery, 2014-2030” report, more than 70% of the products in development are patches incorporating solid or dissolvable needles, the rest are hollow microneedle arrays that employ the use of a syringe. With several new microneedle-based therapeutic product launches by the end of this decade, the report concludes that the overall market for microneedle-based delivery devices will reach annual sales of 485 million units by 2030.
Microneedle-based drug delivery has the potential to be a transformative technology for the delivery of biologics and vaccines. It may provide enhanced therapeutic profiles for therapeutics and vaccines. It allows for the administration of lower levels of drugs to achieve the same therapeutic endpoints. Additionally, microneedles provide an alternative to traditional needles. This industry provides a means to overcome one of the biggest barriers to patient compliance for the treatment of chronic diseases and routine vaccination. The variation in the microneedle types could also prove useful in controlling the kinetics of vaccine release. Such complex variations will further support the use of LSR technology and will be instrumental in the further evolution in the effectiveness and use of transdermal delivery systems.




