Auris Health recently announced FDA clearance for the Monarch Platform, a new robotically-assisted surgical system. Initially cleared for procedures meant to diagnose and treat lung cancer, the system’s approach to flexible technology is being touted as a game-changer by the manufacturer.
In this instance, the company has added historical background that gives their predictions of revolutionary technology a touch more credibility. The CEO of Auris Health is Frederic Moll, MD, who co-founded Intuitive Surgical, manufacturer of the da Vinci.
After leaving Intuitive Surgical, Moll founded Hansen Medical. The company developed the Magellan Robotic System, receiving FDA clearance on the platform in 2012. Designed for cardiac care, the system proved too expensive to compete with the economical cardiac stents that were gaining popularity at about the same time.
When Moll was recruited to the CEO role at Auris Health, he helped shift the company away from a focus on eye surgery — in part because he felt robotically-assisted surgery for those procedures was a tough sell with regulators — and spearheaded the acquisition of Hansen’s technology. It provided the backbone of the Monarch, with the principal clinical use shifting from cardiac care to the treatment of lung cancer.
Moll says the Monarch has the potential to transform the treatment of lung cancer because it provides a minimally invasive means to earlier diagnosis of the disease. The flexible arms of the Monarch can probe more deeply and precisely, and the system combines the video from the endoscopic camera with 3D models of patient anatomy to provide computer-assisted navigation and a uninterrupted view of the entire procedure.
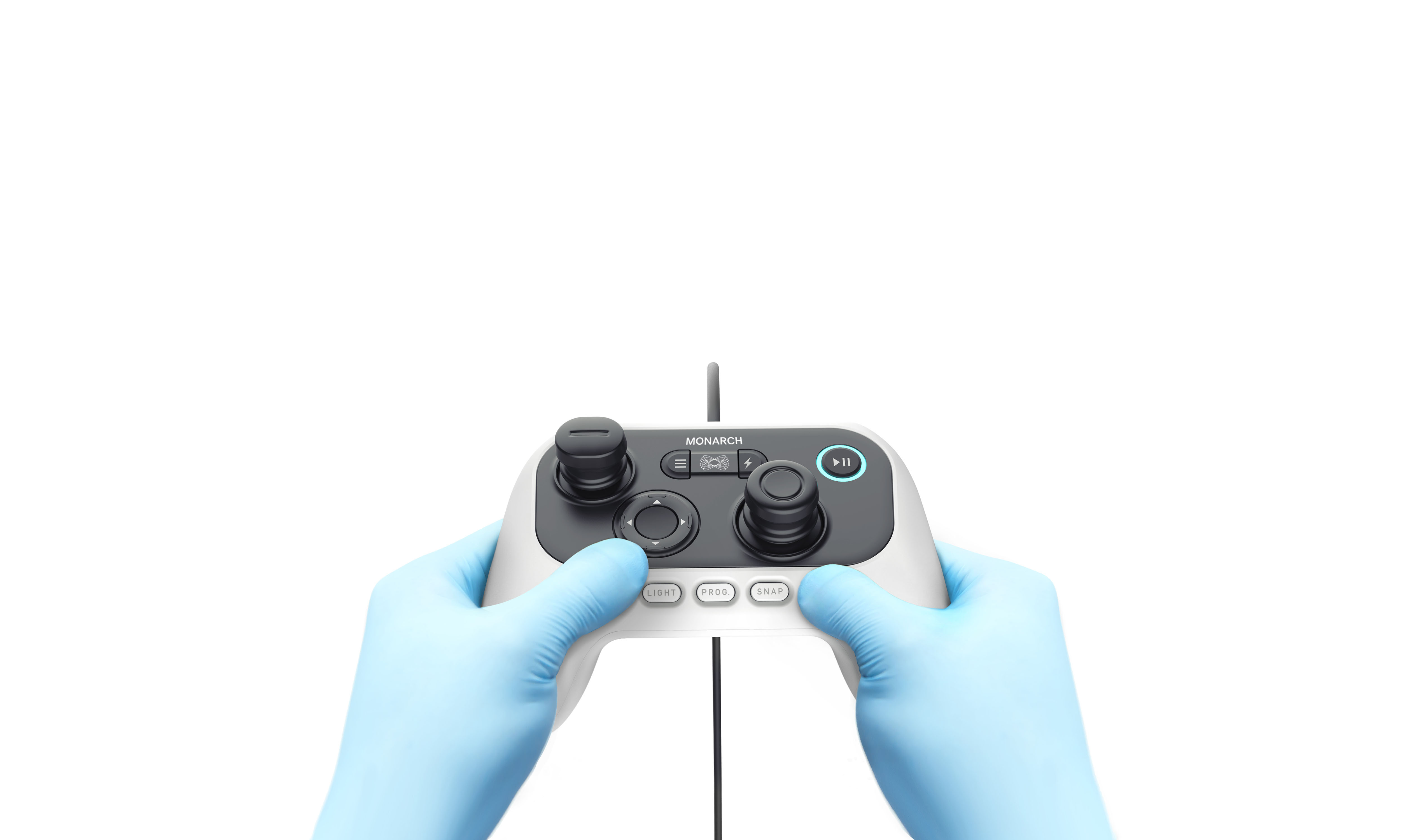
The handheld controller for the Monarch Platform. (Image credit: Auris Health)
“A CT scan shows a mass or a lesion,” Moll explains to TechCrunch. “It doesn’t tell you what it is. Then you have to get a piece of lung, and if it’s a small lesion. It isn’t that easy — it can be quite a traumatic procedure. So you’d like to do it a very systematic and minimally invasive fashion. Currently it’s difficult with manual techniques and 40-percent of the time, there is no diagnosis.”
Moll insists the current FDA clearance is just the beginning, emphasizing the “Platform” in the new device’s name. The intent is for it to eventually be employed for a wide array of endoscopic procedures.
In that longview, Moll and his cohorts also see an even more significant change coming. Although full robotic surgery without any human controls currently remains the stuff of science fiction, Moll predicts the Monarch will eventually perform procedures entirely on its own.
“It’s sort of like self-driving cars. People used to wonder if it was going to be five or 10 years,” Moll tells Bloomberg. “No, no, no: It’s going to be 18 months.”
As for the timing on the Monarch Platform moving fully into the marketplace, Auris is expecting installs of the system in hospitals and other healthcare facilities before the end of the year.




