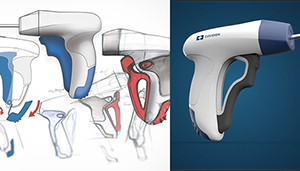
So why does the OR or the medical device field in general need art? Worrell’s founder Bob Worrell lists 2 companies: Apple and Google.
“Wouldn’t think of them as being in the healthcare business, but they are moving into that very significantly,” Worrell said during a recent webinar with MDO. “It’s important, I think, for design to be involved in the healthcare industry, because as this industry grows, it will be crowded. There will be competition. There’s a real need for innovative solutions and points of differentiation, and I think that’s what we designers bring to this program.”
For much of his more than 40 years in healthcare, Worrell answered to engineers. Much of the time, engineers created a device and then asked designers such as Worrell to do a “skin job,” shape the box – basically make it look pretty. Only in recent years have designers been able to bring their “lateral thinking” to bear earlier in the medical device development process, working as partners with engineers.
“We’re generating sketches, we’re generating models, we’re evaluating work flows. It’s really, ‘What part of this product interacts with a human at some point?’ Those are all of the things that we will touch,” said Nicole Parks, industrial design manager at Worrell.
Having a background in a creative field, with a degree in art and design, enables Worrell and Parks to look at things differently. “We’re taught to look at the entire environment that this product is living in,” Parks said. “It helps us think about it in a new light.”
Parks and her colleagues work closely with mechanical engineering staff at Worrell: “We will dream a little big, and the engineers help us make sure it’s realistic at the end of the day and it’s something that can be produced and moved forward with.”
Whether it’s an OR or an ER or a cath lab or another healthcare setting, Worrell designers are trained to very much be a “fly on the wall” and observe work flow. Empathy plays an important role in this process, according to Worrell.
“We see evidence of stress, and different emotional issues that are brought to bear in those environments,” Worrell said. “We’re able to see, ‘Is this tool in its proper context?’ We’re able to see how a device is introduced into the system, we’re able to see what happens to clean up, after this whole event. So we’re bringing our skill sets into this. We’re learning a lot. Then we exit and begin talking to the physicians and the stakeholders in this process.”
The on-site observations prove invaluable when Worrell designers sit down with physicians, mechanical engineers, biomedical engineers and other stakeholders for the brainstorming sessions they call co-creations, Parks said.
“Because I was there, in the observations, I don’t have to ask things like, ‘Well, what would the tray look like that the device is sitting on?’ I know, I can recall that, and so then my sketches reflect those ideas,” Parks said. “And I’m able to add to them. So if surgeons recommend something that they’ve thought of, I can actually add on to that to say, ‘You know I observed this thing as you were doing it. What if we added this feature?’”
“We’ll say, ‘OK, now they have to actuate this button, but they’re not looking at the device because their eyes have to be focused on the patient.’ How are they able to do that? Well, it’s all through touch and feel, and how does the form allow them to grip it in a secure way – while actuating this primary function of the device,” Parks said.
Blood matters, because it’s slippery. “So we’ll mimic it by putting on two layers of latex gloves, which is often what we see in the surgical environment, and we’ll coat our hands with olive oil. That will mimic what a surgeon’s feel is going to be like using this device,” Parks said
As they come up with a host of iterations to consider, exactly how the device is used isn’t the only thing designers are thinking of, either. They’re considering the storage rooms, sterilization processes and even layout of the cables running to and from various devices.
Such lateral, creative thinking is needed because the healthcare industry needs to figure out how to affordably serve a rapidly aging U.S. population, not to mention the billions of people around the world who still do not receive adequate medical attention.
Said Worrell: “It leaves a lot of opportunities for very creative individuals and designers and engineers and imaginative folks to come up with some of these solutions.”
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]