NeuroPace, Inc., a Silicon Valley-based medical technology company, announced that the American Medical Association (AMA) has issued a new Category I Current Procedural Terminology (CPT) code for electrocorticography from an implanted brain neurostimulator. The new code is applicable to services that physicians perform with the company’s RNS System, a first-of-its-kind technology for the treatment of patients with refractory epilepsy.
Beginning January 1, 2019, providers are able to report the new CPT code for the review and interpretation of electrocorticograms (measurements of brain activity) captured and stored by the RNS System. CPT codes are widely recognized by public and private health insurance payers in the United States to describe healthcare services and procedures to facilitate reimbursement. The CPT code application was submitted by the American Academy of Neurology, American Clinical Neurophysiology Society, and the National Association of Epilepsy Centers.
“The support of the medical societies for this newly issued CPT code is a testament to the strength of the long-term clinical evidence supporting this technology as well as the valuable insights from neural recordings that only the RNS System provides,” said NeuroPace CEO Frank Fischer. “The new code should give even greater access to this information and facilitate patient access to RNS therapy.”
Neural data from the RNS System provides information that helps clinicians better understand the location, frequency, and triggers of an individual patient’s seizures, allowing them to personalize care and empowering patients to better manage their condition. Using artificial intelligence and machine learning, NeuroPace scientists are analyzing millions of electrocorticographic recordings to develop algorithms designed to optimize and individualize patient care.
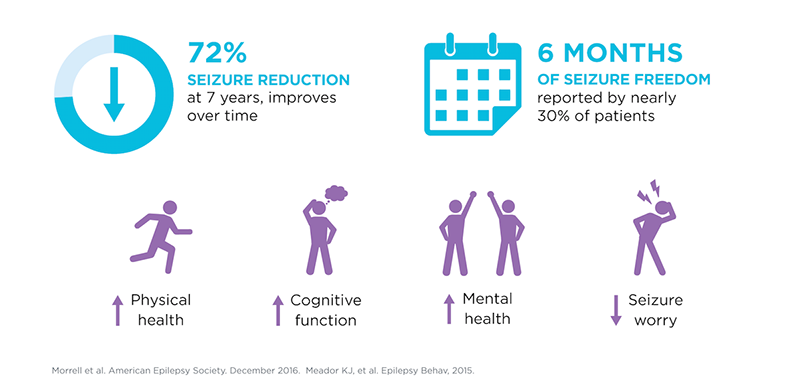
The RNS System treats focal seizures by continuously monitoring brainwaves, recognizing each patient’s unique “seizure fingerprint,” and automatically responding with imperceptible electrical pulses to stop seizures before they occur. Results from a landmark nine-year study of the technology demonstrated that the system resulted in significant seizure reduction, with one in three patients achieving at least 90 percent seizure reduction, and improved quality of life for patients, including cognitive and epilepsy-related measures.1
Of the roughly 3.4 million adults in the U.S. with epilepsy, as many as one-third have seizures that are not controlled by existing therapies. As the only FDA-approved medical device that utilizes brain-computer interface technology for epilepsy, the RNS System offers an advanced treatment option for patients with refractory focal seizures.