A research team centered at Nagoya University developed a device for quick, accurate identification of a mutation strongly associated with a cancer that affects the central nervous system, potentially enabling accurate removal of the entire tumor during an operation.
Gliomas are tumors occurring in the brain or spinal cord. They are difficult to treat as they lack clear edges, which complicates full surgical removal. This leads to high levels of recurrence and mortality. However, previous findings have identified a particular mutation very common in gliomas but rare in other cancers and in normal tissue.
Researchers centered at Nagoya University have now developed a micro-sized device that can determine whether a sample is positive for the mutation using only a small sample. This novel approach takes less than 15 minutes. This potentially allows surgeons to identify the specific type of brain tumor and delineate its margin, in real time during surgery, enabling full removal while sparing normal brain tissue.
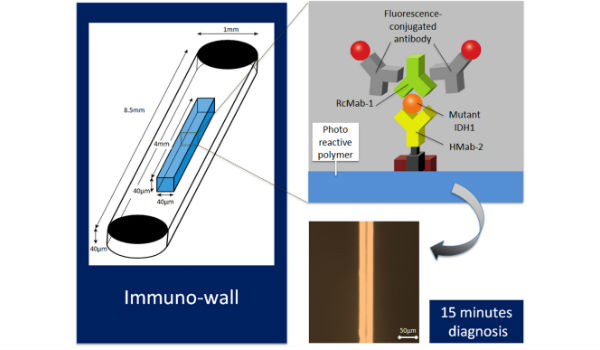
mmuno-wall chips with the photoreactive polymer in the center of the 40 microchannnels are made with a biotinylated anti-R132H-IDH1 antibody (HMab-2), an anti-wild-type IDH1 antibody (RcMab-1), and fluorescent antibodies. It shows sensitive and specific fluorescence from mutant IDH1. (Credit: Nagoya University)
The researchers reported their breakthrough device, which they call an “immuno-wall microdevice,” in Science and Technology of Advanced Materials. The device features a chip with an attached highly specific antibody, HMab-2, produced by Yukinari Kato at Tohoku University. This binds to the protein produced by the gene in which the mutation has occurred. When a sample containing the mutated protein is added to the device, the protein binds to the antibody, which is then specifically detected by a source of fluorescence. In contrast, if the sample is from normal tissue without this mutation, or is from a tumor other than a glioma, no fluorescence occurs.
“The immuno-wall determines whether a sample is positive for a specific mutation in the isocitrate dehydrogenase 1 gene, which is present in around 70–80 percent of grade II and III gliomas,” coauthor Toshihiro Kasama says. “Our results for a range of cancerous cell lines and actual tumor samples both positive and negative for this mutation were very promising.” The device was proven highly accurate, as confirmed by complete sequencing of the gene in question in each sample.
The small sample size required for the device reduces the invasiveness of sample harvesting. In fact the process takes only 15 minutes, enabling completion during an operation. The immuno-wall could markedly increase success of glioma treatment by rapidly providing data to inform the course of the operation and tissue to remove.
“Our data indicate that a sample with just 500 cells or 500 ng of protein is sufficient to give a positive result,” lead author Akane Yamamichi says. “The key to success in the immuno-wall assay is that we, luckily, have HMab-2, the highly specific antibody to the mutant IDH1. This means the immuno-wall can identify the margins of tumors where only low numbers of cancerous cells are present.”
Alternatively, sampling could even involve only obtaining blood or cerebrospinal fluid, rather than removing brain tissue, making the procedure even less invasive.
(Source: AlphaGalileo)
