Researchers at School of Medicine and three hospitals in Chile have demonstrated the safety of a new magnet-driven device that enables surgeons to make fewer incisions while performing laparoscopic gallbladder surgery.
Each year, more than 1 million people in the United States have their gallbladders removed, putting that procedure, called a cholecystectomy, on the nation’s top-10 list of surgeries. The gallbladder is closely sheltered in the curve of the liver, so removing it can be tricky. Even in the most skilled hands, manipulating the laparoscopic instruments used in the surgery poses a risk of causing internal damage that can lead to scarring.

A photo of the magnet-driven tool, which has received clearance by the Food and Drug Administration. (Crtedit: Levita Magnetics)
The new magnet-driven device makes the surgery less invasive by obviating the need for an incision through which an instrument is inserted to retract the gallbladder. Instead, an external magnet does that job.
In a paper published online Oct. 24 in Annals of Surgery, the researchers shared the results of a 50-patient clinical trial in which they demonstrated the safety of the device. Homero Rivas, MD, assistant professor of surgery at Stanford and director of innovative surgery at Stanford Health Care, is lead author of the paper. The senior author is Mario Uribe, MD, of the Hospital Salvador in Santiago, Chile.
Nationwide, the device, which has received clearance from the Food and Drug Administration, is in use at Stanford Medicine and two other medical centers.
“Laparoscopy has truly revolutionized surgery over the past 30 years or so, and with very good results, but it still relies on a given number of incisions and instruments,” Rivas said. “Surgeons and patients continuously search for painless, scarless operations. This device takes a step toward that goal by reducing the need for fixed, transabdominal instruments.”
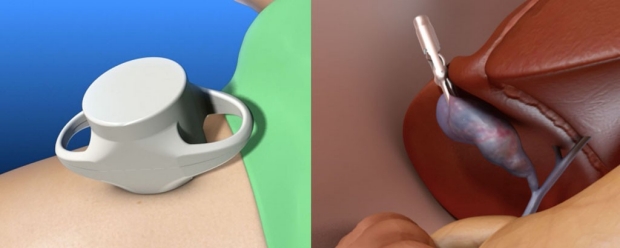
In an illustration of how the tool works, the external magnet is depicted on the abdomen (left), and the grasper, which is attracted to the magnet, is attached to the gallbladder (right). (Credit: Levita Magnetics)
How it works
The device has two parts: an external magnet and a slender rod with a detachable clip that includes a magnet. The rod, with the clip at its far end, looks much like the reaching devices that people can use to grab objects from high shelves. A surgeon inserts the rod through the belly button and manipulates its clip to grasp the gallbladder. The clip is then released from the rod but remains connected to the gallbladder. The external magnet is placed on the abdomen to control the movement of the clip attached to the gallbladder. A rod with a camera attached to it sends video to a monitor in the operating room, giving the surgeon an inside view of the area near the gallbladder. Other instruments are inserted through incisions to detach the organ, after which the rod that was earlier unhitched from the clip is reconnected to it and used to remove the organ from the patient.
The study documented an average hospital stay for patients of 22 hours, and an average pain score of 0.6 on a scale of 0 to 10 seven days after surgery. The average time for patients to return to work was five days.
The device was the idea of Alberto Rodriguez-Navarro, MD, who specialized in minimally invasive surgery in his native Chile and is now CEO of Levita Magnetics, the San Mateo-based company he founded to develop the magnetic surgical system.
Rivas said he has used the device primarily for cholecystectomies, but he believes it is versatile enough to be applied to bowel resections, appendectomies, hysterectomies, gastrectomies and other abdominal surgeries. “I hope that greater availability of the device will allow other innovators to propose other uses that even its pioneers have not thought of,” Rivas said.
Surgeons at three Santiago hospitals — the Hospital Salvador, the Hospital Luis Tisne and the Hospital Padre Hurtado — also are co-authors of the study.
The research was supported by a grant from the Chilean Economic Development Agency and sponsored by Levita Magnetics.




