Mechanical engineering researchers have developed a method that could extend the life of an artificial hip by adding an array of microscopic indentations that increase the thickness of a lubricating film on its surface.
“Lubrication plays a key role in the wear process of the implanted joint interface,” says Min Zou, professor of mechanical engineering. “But this mechanism is challenging to understand because of the complex nature of the viscous fluid used for lubrication. Our study reveals promising results for understanding this mechanism better and significantly improving implants.”
Each year in the United States, orthopedic surgeons perform more than 300,000 hip-replacements, including roughly 36,000 revision surgeries when a replacement wears out. Though there are several environmental factors that affect the health and longevity of artificial hips, these implants last an average of 15 to 20 years. However, about half of all hip-replacement surgeries are performed on patients between the ages of 45 and 64, people who could live another 25 or 30 years.
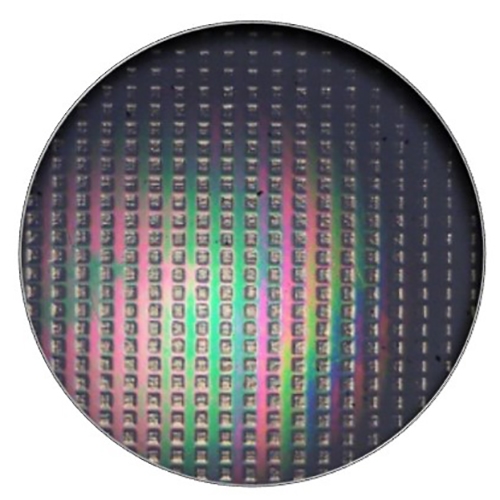
An array of square dimples on the surface of artificial femoral heads enhance the thickness of the lubricating film that forms between the interacting surfaces of artificial hip implants. Photo provided by the researchers. (Image credit: University of Arkansas)
Hip-replacement procedures involve the surgical removal and artificial replacement of the femoral head – the top of the femor, or thigh bone – and part of the pelvis called the acetabulum, a concave surface into which the femoral head fits. Many people refer to this joint as a ball and socket – the femoral head acting as the ball and the acetabulum as the socket.
A primary reason for artificial joint failure – and thus the need for revision surgery – is a loosening of the bond between the implant and bone, which in many cases is caused by the body’s autoimmune response to particles and wear on the surface of the implant, as well as the toxicity of metallic ions released into the body because of this wear.
To address this problem, researchers focus on a lubricating film that forms between the interacting surfaces of the femoral head and the acetabulum. This film is created by a naturally occurring lubricant called synovial fluid, which is produced and released by cartilage. Although cartilage is also removed during hip-replacement surgery, the body produces enough synovial fluid to form a thin layer between the implants. The formation of this film after surgery and especially its thickness determine the long-term success of the implant.
The U of A experiment was designed by Dipankar Choudhury, post-doctoral fellow in the Center for Advanced Surface Engineering, and performed at the direction of Zou, who is director of the center. Their results were published in the Journal of the Mechanical Behavior of Biomedical Materials.
Rather than manipulating synovial fluid, which most hip prosthesis research focuses on, Zou and Choudhury simulated the textured surface of cartilage, its so-called “converging gaps,” or natural dimples, that promote the secretion of synovial fluid and enhance thickness of the lubricating film.
Zou and Choudhury designed square-, triangular- and elliptical-shaped indentations on the surface of artificial femoral heads. (Magnified images resemble the surface of a golf ball.) These prostheses were made of chromium, cobalt and molybdenum, the standard metallic alloy for hip-replacement implants. The indentations, fabricated with a picosecond laser by a collaborator in Japan, were 20 to 50 micrometers wide and 0.2 to 1 micrometer deep.
Then, collaborating with a research group in the Czech Republic, Zou and Choudhury joined the prosthetic parts and performed wear and friction tests with a pendulum that simulated the swinging motion of a human leg. When the pendulum returned to equilibrium position – after the lubricant film had fully developed – test results revealed significantly enhanced thickness of film. Compared to the non-dimpled prostheses, all three shapes improved lubricant film thickness. The prostheses with square- and triangular-shaped indentations demonstrated an average lubricant film thickness 3.5 times greater than the non-dimpled prosthesis.
The prosthesis with square-shaped indentations formed its lubricant film less than a second after the pendulum started moving, much faster than all other prostheses, including those with indentations made of other shapes. This clearly demonstrated that shape had a significant effect, Choudhury says.
“Also, hardly any scratches appeared on the post-experimental prosthesis with square-shaped dimple arrays, and only a few scratches were found on the prosthesis with triangular-shaped dimple arrays,” says Zou. “We think that prostheses with square-shaped dimples could be a potential solution for durable, longer-lasting artificial hip joints.”
Zou recently developed processes for fabricating smaller micro-dimples of various complex shapes, which are expected to further decrease friction and wear of the prosthetic joints.
Zou holds the Twenty-First Century Professorship in mechanical engineering. In 2015, she received a $20 million National Science Foundation/EPSCoR (Experimental Program to Stimulate Competitive Research) grant and an additional $4 million matching funds from the state, which enabled the university to establish the statewide Center for Advanced Surface Engineering. The center promotes statewide scientific progress by establishing partnerships with government, higher education and industry.




