Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and UC Berkeley have created a sort of nanoscale display case that enables new atomic-scale views of hard-to-study chemical and biological samples.
Their work could help to reveal new structural details for a range of challenging molecules — including complex chemical compounds and potentially new drugs — by stabilizing them inside sturdy structures known as metal-organic frameworks (MOFs).
The researchers introduced a series of different molecules that were chemically bound inside these porous MOFs, each measuring about 100 millionths of a meter across, and then used X-ray techniques to determine the precise molecular structure of the samples inside the MOFs.
The samples ranged from a simple alcohol to a complex plant hormone, and the new method, dubbed “CAL” for covalent alignment (the molecules form a type of chemical bond known as a covalent bond in the MOFs), enables researchers to determine the complete structure of a molecule from a single MOF crystal that contains the sample molecules in its pores.
The MOFs in the study, which are identical and are easy to manufacture in large numbers, provided a sort of backbone for the sample molecules that held them still for the X-ray studies—the molecules otherwise can be wobbly and difficult to stabilize. The researchers prepared the samples by dipping the MOFs into solutions containing different molecular mixes and then heating them until they crystallized.
“We wanted to demonstrate that any of these molecules, no matter how complex, can be incorporated and their structure determined inside the MOFs,” said Omar Yaghi, a materials scientist at Berkeley Lab and chemistry professor at UC Berkeley who led the research.
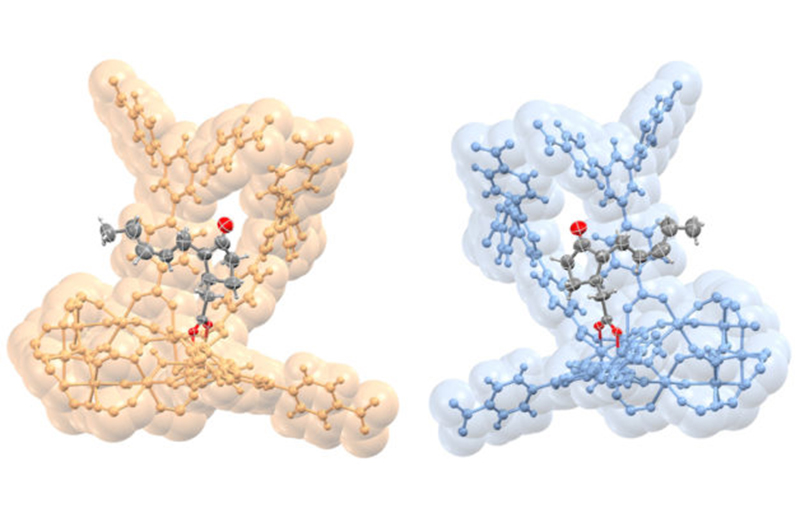
This rendering shows opposite configurations in the molecular structure of a plant hormone called jasmonic acid (gray and red) that are bound to nanostructures (gold and blue) called MOFs, or metal-organic frameworks. (Credit: S. Lee, E. Kapustin, O. Yaghi/Berkeley Lab and UC Berkeley)
The MOFs also possess a particular handedness known as “chirality” — like a left-handed person vs. a right-handed person — that selectively binds with molecular samples that also possess this handedness. The difference in a molecule’s handedness is particularly important for pharmaceuticals, as it can mean the difference between a medicine and a poison.
One of the best methods for studying any molecule’s 3-D structure in atomic detail is to form it into a crystal. Then, researchers point intense X-ray light at the crystal, which produces a pattern of spots—like light off of a disco ball. Such patterns serve as a fingerprint for fully mapping the molecule’s 3-D structure.
Some molecules are difficult to form into crystals, though, and the process of crystallizing a single molecule can in some cases involve years of effort and expense.
“To crystallize a molecule typically involves a trial-and-error method,” Yaghi said. “Every chemist and biologist has to submit to this process. But in this MOF material you don’t need all that—it traps the molecule and orders it. It’s a way to bypass that trial-and-error approach to crystallography.”
Importantly, the MOFs in the latest study did not appear to distort the natural, intact structure of the molecules. Researchers say it’s possible to determine the complete 3-D structure of a molecule even if the samples only fill about 30 percent of a MOF’s pores.
In all, the researchers studied 16 different molecules bound inside the MOF pores, including a plant hormone called jasmonic acid whose chiral structure had never been directly determined before, other plant hormones known as gibberellins, methanol, and other acids and alcohols.


