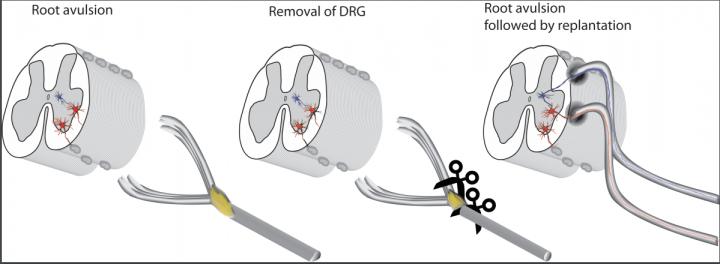
Both ventral and dorsal roots have been detached, which is the common scenario clinically, the ventral root is implanted directly into the cord as is dorsal root, but after the ganglion has been cut out. In the final drawing the possible re-established connections and reflex arch are colored. (Credit: Thomas Carlstedt and Mårten Risling)
Scientists in the UK and Sweden previously developed a new surgical technique to reconnect sensory neurons to the spinal cord after traumatic spinal injuries. Now, they have gained new insight into how the technique works at a cellular level by recreating it in rats with implications for designing new therapies for injuries where the spinal cord itself is severed.
The brain and the neurons (nerve cells) in the rest of our body are connected in the spine. Here, motor neurons, which control muscle movement, and sensory neurons, which relay sensory information such as pain, temperature and touch, connect with the spinal cord.
Where the neurons connect with the cord, motor neurons bundle together to form a structure called the motor root, while sensory neurons form a sensory root. In patients with traumatic injuries, these roots can be torn, causing areas of the body to lose neural control.
Surgeons can implant motor roots at the area from which they are torn, and they will usually successfully reconnect, as motor neurons can regrow out of the spinal cord and into the motor root. However, this does not apply to the more troublesome sensory root, which surgeons couldn’t reconnect properly until recently. “Doctors previously considered this type of spinal cord injury impossible to repair,” says Nicholas James, a researcher at King’s College London. “These torn root injuries can cause serious disability and excruciating pain.”
Happily, Thomas Carlstedt, also at King’s College London, recently helped to develop a new surgical technique to reconnect the sensory root. It involves cutting the original sensory nerve cells out of the root and implanting the remaining root directly into a deeper structure in the spinal cord. This area is called the dorsal horn, and it contains secondary sensory neurons that don’t normally directly connect to sensory roots. When the team tried the technique in patients, certain spinal reflexes returned, indicating that the implanted neuron had integrated with the spine to form a functional neural circuit.
In a new study recently published in Frontiers in Neurology, James, Carlstedt and other collaborators set out to understand how the implanted sensory root was connecting with the spinal cord in the dorsal horn. By understanding the mechanism, they hope to develop new treatments for patients with other types of spinal injuries.
The scientists used a rat model of spinal injury to study the process at a cellular level. During surgery, they produced a similar spinal injury in the rats and then reattached the sensory root using the new technique. At 12-16 weeks after surgery, the researchers assessed the spinal repair by passing electricity along the neurons to see if they formed a complete neural circuit. They then sacrificed the rats and analyzed the neural tissue under a microscope.
The electrical tests showed that the neural circuit was complete, and that the root had successfully integrated with the spinal cord. When they examined the tissue, they found that small neural offshoots had grown from structures called dendrites (branched projections at the end of neurons) in the dorsal horn. These thin offshoots had extended all the way into the implanted sensory root to create a functional neural circuit.
So, what does this teach us about spinal cord repair? The researchers hope that this type of neural growth could also be used to repair other types of spinal cord injury. “The strategy of encouraging new growth from spinal neurons could potentially be of use in other injuries of the nervous system,” says Carlstedt. For example, scientists could capitalize on this mechanism when designing new therapies for injuries where the spinal cord itself is severed, by implanting grafts that encourage or facilitate this type of nerve growth.




