The Edwards Intuity Elite valve system received U.S. Food and Drug Associaation (FDA) clearance this week, more than two years after it was approved for use in Europe. In a press release issued by Edwards Lifesciences, the California company’s corporate vice president Bernard Zovighian enthuses, “U.S. approval of the Edwards Intuity Elite valve system is a significant milestone as this technology provides an advanced surgical treatment option for patients suffering from aortic valve disease.”
Built on the longstanding Perimount heart valve design, the Edwards Intuity is touted as “a rapid deployment device for surgical aortic valve replacement.” Company literature emphasizes the device’s secure assembly, rapid valve preparation, and design incorporating the balloon within the delivery system as elements that will lead to more efficient procedures. They also promise smaller incisions due to its reliance on only three guiding sutures, instead of the 12-15 used by competing devices.
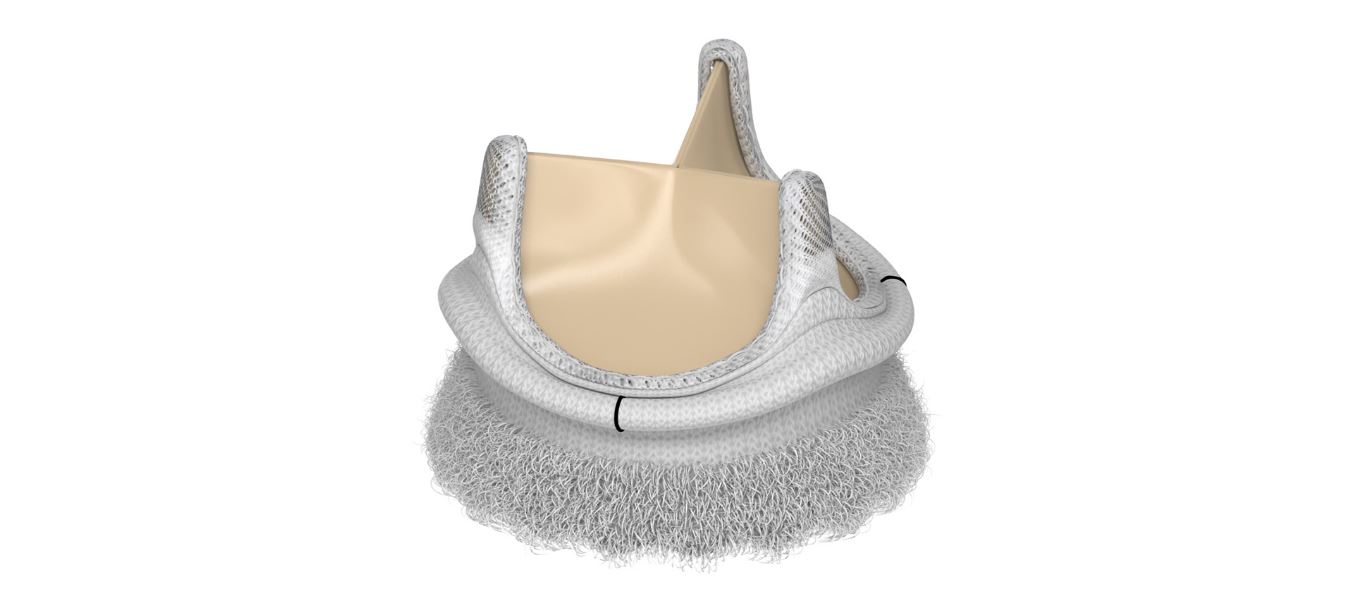
(Image credit: Edwards Lifesciences)
Kevin Accola, M.D., program director at the Valve Center for Excellence at the Florida Hospital Cardiovascular Institute in Orlando, Flordia, weighed in for Edwards, explaining, “Many patients with aortic stenosis have more than one type of heart disease, most commonly coronary artery disease. The Edwards Intuity Elite valve system enables surgeons to streamline concomitant procedures, which can be beneficial for patients undergoing these longer, more complex surgeries,”
Edwards Lifesciences reports the device is immediately available commercially in the U.S.




