 PathMaker Neurosystems announced this week that it has established a research relationship with the Brain and Spine Institute (Institut du Cerveau et de la Moelle Epinière, or ICM) at the Pitié-Salpêtrière Hospital in Paris. The institute will carry out the clinical trials that PathMaker needs to achieve CE Mark for its MyoRegulator system.
PathMaker Neurosystems announced this week that it has established a research relationship with the Brain and Spine Institute (Institut du Cerveau et de la Moelle Epinière, or ICM) at the Pitié-Salpêtrière Hospital in Paris. The institute will carry out the clinical trials that PathMaker needs to achieve CE Mark for its MyoRegulator system.
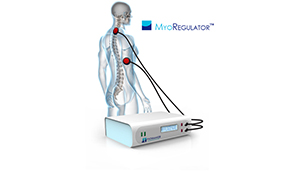
Boston-based PathMaker boasts that the MyoRegulator is the first noninvasive neuromodulation device to treat people with neuromotor spasticity.
The company expects to start formal clinical trials in France later this year, pending approval by the French National Institute of Health and Medical Research. The MyoRegulator is already under investigation in Institutional Review Board–approved clinical trials.
The Brain and Spine Institute is a French center of excellence, with more than 600 physicians and scientific researchers focused on neurological diseases. PathMaker is a member company of the iPEPS-ICM bioincubator, meant to accelerate breakthrough neuroscience ideas into innovative products.
“We are very pleased to be broadening our relationship with ICM,” Dr. Nader Yaghoubi, PathMaker’s president and CEO, said in a news release.
“With the agreements that we have recently put in place, we will be working with ICM to carry out European clinical trials for our breakthrough neuromodulation technology,” Yaghoubi said. “This important collaboration establishes a significant cornerstone of our Company’s trans-Atlantic strategy—gaining access to ICM’s leading specialists and researchers in neuroscience, access to the specialized Clinical Investigation Centre at ICM, and obtaining exclusive rights to technology emerging in our field from ICM.”
[Want to stay more on top of MDO content? Subscribe to our weekly e-newsletter.]




