Novo Nordisk Inc. is recalling six batches of the GlucaGen HypoKit in the U.S. due to two customer complaints from the UK and Portugal involving detached needles on the syringe with Sterile Water for Injection (SWFI). GlucaGen HypoKit is indicated for the treatment of severe hypoglycemia (low blood sugar) in patients with diabetes who are treated with insulin. A syringe with a detached needle cannot be used as prescribed.
Untreated hypoglycemia can eventually lead to unconsciousness and seizures, which can prove fatal. If the blood glucose levels are not quickly restored, continuing hypoglycemia can lead to a decline in brain glucose levels which manifests through a variety of symptoms including cognitive dysfunction, sweating, tremors, convulsion and eventually coma or death.
Novo Nordisk conducted an investigation which showed that a small number (0.006 percent) of needles could be detached from the syringe in certain batches of GlucaGen HypoKit. To protect patient safety, Novo Nordisk is recalling affected batches from wholesalers, pharmacies and patients in the U.S. It is estimated that out of the 71,215 pens being recalled, four pens could be defective.
Patients or caregivers should check the batch number to see if their GlucaGen HypoKit is affected. The batch number is printed on the GlucaGen HypoKit as indicated below in the red box (Figure 1).
This recall includes GlucaGen HypoKit batch numbers:
- Batch: FS6X270, Expiry: 09/30/2017
- Batch: FS6X296, Expiry: 09/30/2017
- Batch: FS6X538, Expiry: 09/30/2017
- Batch: FS6X597, Expiry: 09/30/2017
- Batch: FS6X797, Expiry: 09/30/2017
- Batch: FS6X875, Expiry: 09/30/2017
The affected products were distributed starting February 15, 2016.
Novo Nordisk is working as quickly as possible and in collaboration with the U.S. Food and Drug Administration (FDA) to recall affected products from the marketplace, including those in the possession of patients. To date, Novo Nordisk is not aware of any known adverse events resulting from the use of the recalled batches.
The batch number is printed on the GlucaGen HypoKit as indicated below.
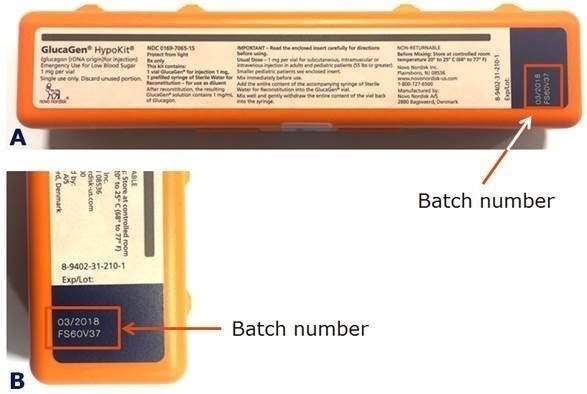
A) GlucaGen HypoKit where the batch number is found in the red box, B) close up of the batch number
If you have a GlucaGen HypoKit with one of the above-mentioned batch numbers, call 1-888-840-1137 from Monday to Friday, between 8:30am – 6:00pm Eastern Time, to find out how to return the product. Novo Nordisk will provide reimbursement for out-of-pocket costs incurred for the purchase for your affected GlucaGen HypoKit with proof of purchase. If you received a GlucaGen HypoKit through the Novo Nordisk Patient Assistance Program, you will receive a replacement device.
If you are in possession of a GlucaGen HypoKit with a batch number NOT mentioned above, the product is not subject to the recall and may be used as prescribed.
Novo Nordisk Inc. is notifying its distributors and customers by letter and phone and is arranging for return of all recalled products.
Adverse reactions or quality problems experienced with the use of this product may be reported to Novo Nordisk by calling 1-800-727-6500. Patients can also call the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
GlucaGen HypoKit is a prescription medicine used to treat very low blood sugar (severe hypoglycemia) in people with diabetes who use insulin.
About Novo Nordisk
Novo Nordisk is a global healthcare company with more than 90 years of innovation and leadership in diabetes care. This heritage has given us experience and capabilities that also enable us to help people defeat other serious chronic conditions: hemophilia, growth disorders and obesity. With U.S. headquarters in Plainsboro, N.J., Novo Nordisk Inc. has more than 5,000 employees in the United States. For more information: www.novonordisk.us.
(Source: PR Newswire)




