Earlier this year, Olympus America secured FDA 510(k) clearance for their next-generation laparoscopic PK Morcellator. That was the final piece needed to pull together a new, complete solution for laparoscopic tissue containment and extraction.
Primarily comprised of the PK Morcellator and a unique containment device called the PneumoLiner, Olympus’s new Contained Tissue Extraction System gives physicians a minimally invasive option for certain patients seeking treatment for uterine fibroids.
To learn more, Surgical Products interviewed Manisha Shah-Bugaj, vice president of marketing, gynecology, and market development at Olympus.
What can you tell us about the new system Olympus has developed?
The Contained Tissue Extraction System from Olympus is the first complete system designed for contained tissue extraction to enter the U.S. market, enabling surgeons to isolate and perform uterine tissue morcellation and extraction using the PneumoLiner containment device.
The PneumoLiner is currently the first and only containment device to receive FDA market approval for use with certain laparoscopic power morcellators to isolate uterine tissue that is not suspected to contain cancer. And once it’s insufflated, the PneumoLiner allows speed and vision throughout the power morcellation procedure while also maintaining a barrier to the escape of fluids, cells, and tissue fragments.
Olympus worked closely with physicians and designers on the system, always with patient safety as a top priority. During that process, our goal was to further mitigate the risks associated with undetected uterine sarcoma. The system provides a complete solution for contained tissue extraction, ultimately providing surgeons and patients a choice when determining the best treatment option. This system really provides certain patients a laparoscopic surgery option to avoid open hysterectomy and myomectomy for uterine fibroid removal.
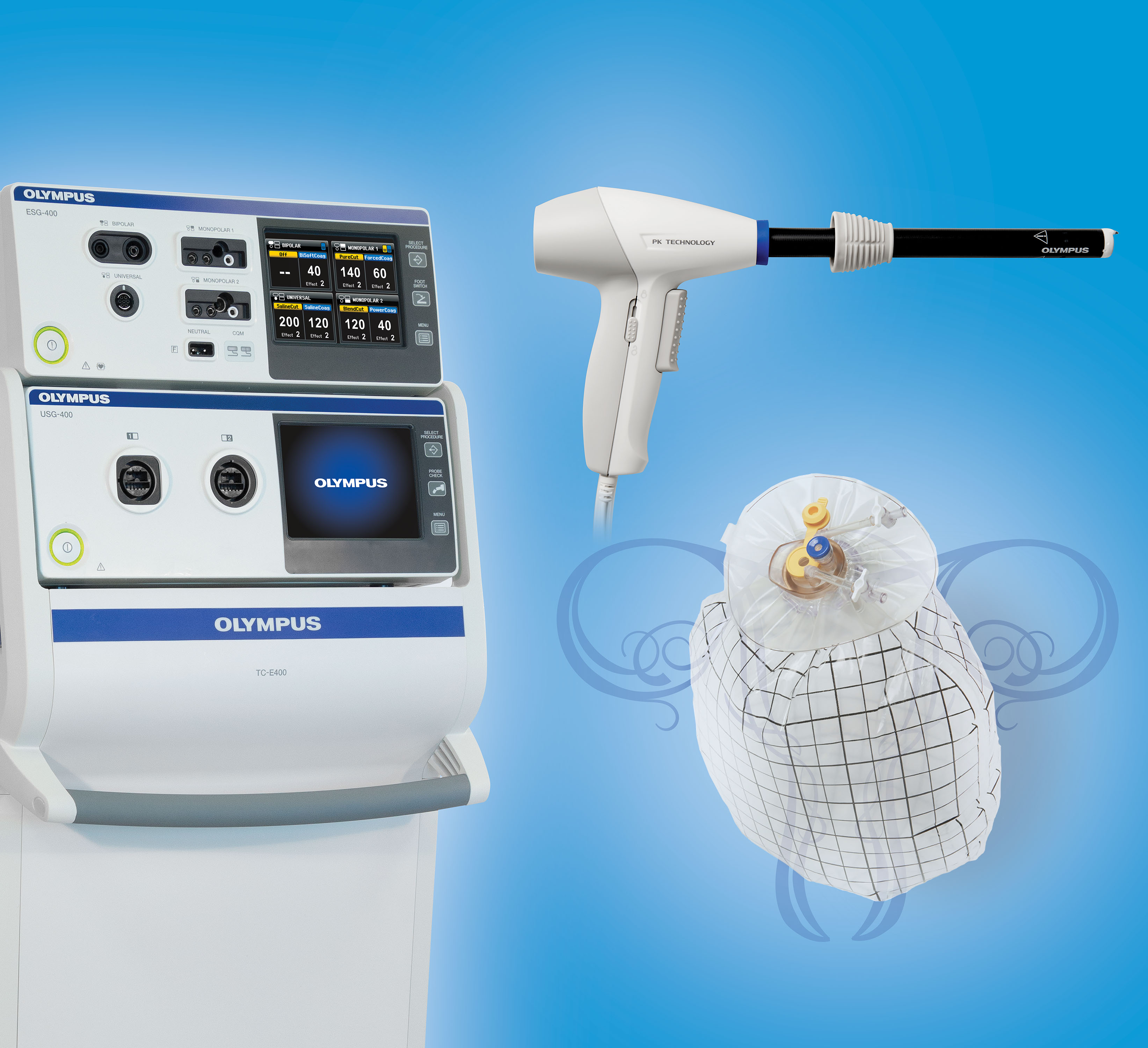
(Image credit: Olympus)
How did the recent concerns about the potential risks of power morcellation influence the development of this new device?
I think as a result of some of the controversies around power morcellation, we’ve seen an increase in open surgery, and a lot of professional societies — like ACOG, AAGL, SGO — have openly voiced concerns about exposing a majority of women who have benign fibroid disease to risks and complications associated with open procedures. And so this new containment system is really an innovation to restore the benefits of minimally invasive surgery.
For appropriate low risk patients this gives them an option to have a minimally invasive approach.
What are the patient populations that are or aren’t well-suited for the new device?
This solution is not appropriate for peri- or postmenopausal women who have suspected fibroids. Also, it’s not for patients who are suspected or known to have cancer.
But there are a population of women who have hysterectomies for indications other than fibroids such as abnormal uterine bleeding, endometriosis, adenomyosis, pelvic pain — they may be appropriate candidates, based on their age. Also, it’s well-suited for women who are potentially having challenges getting pregnant, or a younger population who could be candidates for a laparoscopic myomectomy. This would allow them to have an option to avoid an open procedure.
What education efforts will you have in place?
Our first approach to the market entry is really focusing on surgeon training. For the PneumoLiner in particular, there is an FDA requirement for us to provide training prior to clinical use of the device. So that is really the initial focus of our education on the technology, at the clinician level. And then ultimately with all procedures and treatment options, we want the clinician to be able to educate and provide informed consent to their patients to determine what the best treatment option is for the individual patients.
What sort of feedback have you been getting from surgeons thus far?
I think there’s been a lot of interest from the surgeon community for this technology, because they do see there is a population of women who are good candidates for this. The formal, FDA-required training program was launched in November, 2016. And the training protocol was actually developed and validated by the surgeon community.
There’s increasingly a preference in the marketplace for wide-ranging, comprehensive solutions. How is Olympus meeting that need?
Olympus has demonstrated, even to date, an ability to have a larger impact on our customers through some of our technologies. One of the things we’re very proud of is that we try to build universal platforms. Whether you look at our imaging technologies or our electrosurgical generator — these are technologies that can be used across specialities, for multiple applications, and they can provide operational efficiency, financial savings, and have more of an impact than just a one-off product solution.




