(Credit: OrbusNeich)
OrbusNeich, a company specializing in the provision of life-changing vascular solutions, has announced the launch of its Sapphire PTCA balloon dilatation catheters following their recent 510k clearance by the FDA: the Sapphire II PRO and the Sapphire NC Plus.
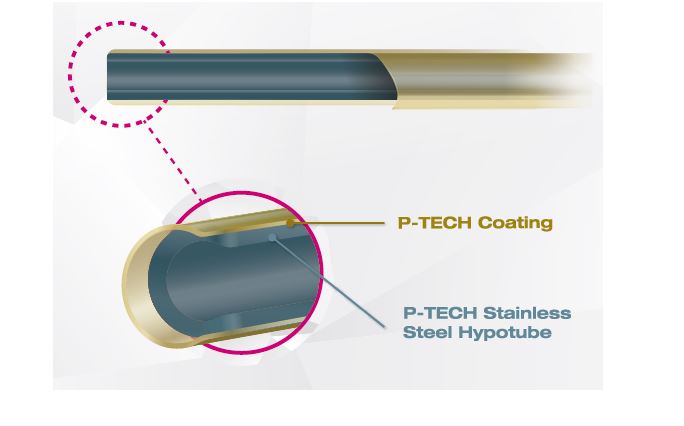
Specifically engineered for crossing the most difficult lesions and tracking tortuous anatomy, the Sapphire II Pro is tailored for successful dilatation. Its well-balanced sub-zero tapered tip has an ultra-low profile, providing effortless entry through the tightest lesions. Other features include a proprietary XR balloon for best-in-class crossability and recrossability without compromising durability and robustness.
Sapphire NC Plus a true non-compliant balloon featuring TiFo (tight fold) folding of the balloon material for enhanced crossability in the tightest lesions, Hydro-X coating for improved lubricity and passability, and an enhanced distal tip which allows for smooth lesion entry.
Both products are indicated for a variety of uses including:
- balloon dilatation of the stenotic portion of a coronary artery or bypass graft stenosis in patients evidencing coronary ischemia for the purpose of improving myocardial perfusion, and
- balloon dilatation of a coronary artery occlusion for the treatment of acute myocardial infarction
In addition, the Sapphire NC Plus is further indicated for dilatation of in-stent restenosis and post-delivery expansion of balloon expandable coronary stents.
“The Sapphire II Pro and Sapphire NC Plus are tried and proven technology in both the interventional coronary and interventional radiology markets outside the US. Entering the US market with our proven coronary dilatation catheters is a long overdue and logical step for our company,” says Scott Addonizio, Chief Operating Officer, OrbusNeich. “Since our establishment in 2005, we have delivered an extensive portfolio of unique products that have changed the lives of patients and their families around the world. Our focus will now include the US market and we are confident our product will be well received.”
A long-time leader in coronary artery disease treatment, in 2013 OrbusNeich launched the innovative COMBO Dual Therapy Stent, the world’s first DES with the unique biological solution for active healing to accelerate endothelial coverage and control neointimal proliferation through the combination of the proven Pro-Healing Technology with an abluminal sirolimus drug elution delivered from a bioresorbable polymer that is completely dissipated within 90 days. In November 2016 OrbusNeich launched the new generation COMBO Plus in select countries. COMBO is supported by clinical evidence from the REMEDEE family of studies and several other investigator-initiated studies, totaling over 6,000 patients enrolled across more than 26 countries.




