OrthoXel is recently announced that the new Apex Femoral Nailing System has been granted FDA 510(k) clearance, following regulatory clearances and first clinical implantations of the Apex Tibial Nailing System earlier this year.
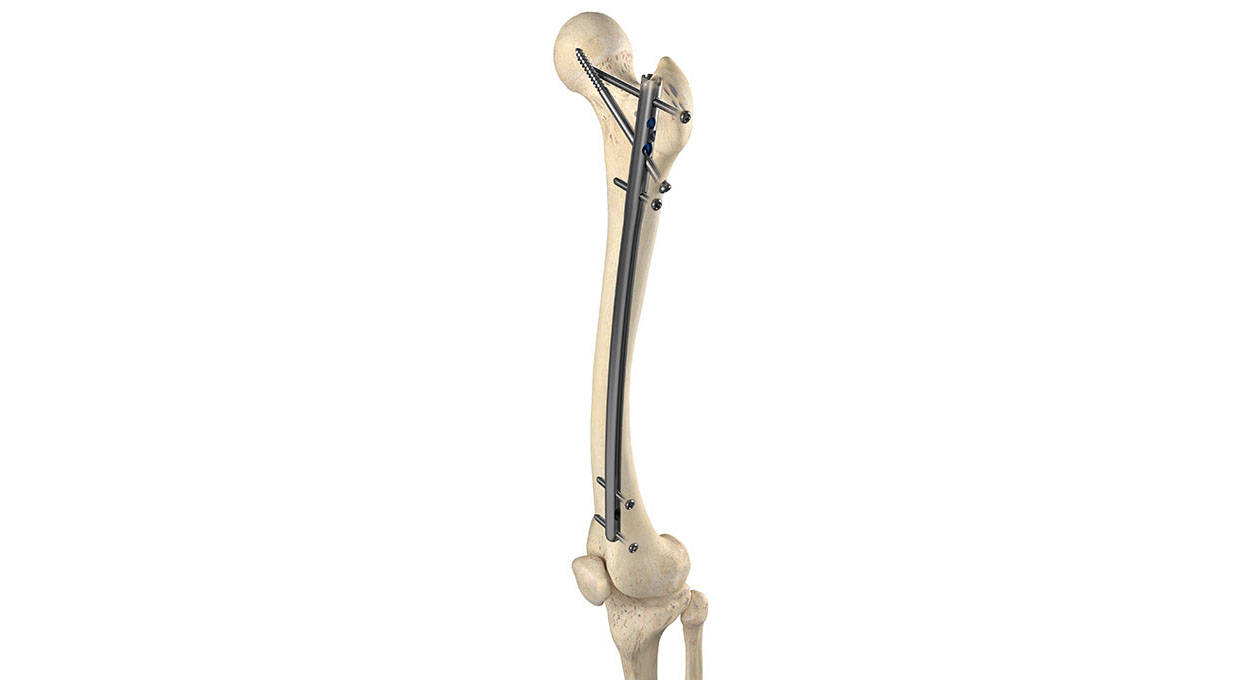
The Apex Femoral Nailing System features a modern anatomic nail curvature in a universal nail that can be surgically implanted from antegrade or retrograde orientations with a dedicated instrumentation kit. The system offers a comprehensive suite of versatile multiple-trajectory locking options including patented OrthoXel micromotion for controlled axial movement with exceptional torsional stability to promote callus formation. Additional locking options include recon and rigid interlocking for unstable proximal femoral fractures.
(Image credit: OrthoXel)
The unique Apex Femoral Nail locking options provide ultimate flexibility and control for the surgeon to choose the right fixation for each patient. In addition to patented gliding micromotion, the Apex system offers an innovative locking endcap that can simultaneously lock multiple bone screws for added stability when needed.
- Micromotion Locking: Up to two mediolateral bone screws and one recon bone screw provide torsional stability with patented controlled axial micromotion.
- Recon Rigid Locking: Up to two mediolateral bone screws and two recon bone screws provide rigid locking, with an optional all screw locking endcap.
- Rigid Interlocking: Up to two mediolateral bone screws, one recon bone screw, and one interlocking bone screw provide rigid locking. The optional addition of the locking endcap simultaneously locks all four screws when needed.
“While we recognize the challenge of entering markets dominated by large multinationals, OrthoXel is confident that the unique locking options and advantageous biomechanics of both our Apex Femoral and our Apex Tibial Nailing Systems confers real market advantage,” says Pat O’Connor, co-founder and CEO of OrthoXel. “Our products really should be the devices of choice for orthopedic trauma care.”




