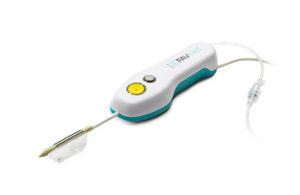
(Image courtesy of PAVmed)
PAVmed (NSDQ: PAVM) today announced that it has received the CE mark for its CarpX minimally invasive carpal tunnel device.
EU-based Notified Body TÜV Rhineland LGA Products GMBH issued CE certificate, effective May 24, 2021, the company noted. CarpX may now be marketed in CE Mark European countries, which include the European Economic Area (the EU, Norway, Iceland and Lichtenstein), Switzerland, and, until July 1, 2023, the U.K.
“This important milestone is a testament to the tenacity, skill and hard work of our team, led by our director of duality, Matt Ennis,” said PAVmed CEO Dr. Lishan Aklog in a news release. “Carpal tunnel syndrome is widely prevalent in Europe and the estimated total addressable market opportunity for CarpX there is at least as large as in the U.S. We look forward to moving towards commercial launch in select European countries in the near future.”
CarpX is a percutaneous device designed to allow the operating physician to relieve compression of the median nerve without a surgical incision. The system combines a balloon catheter with bipolar radio-frequency cutting electrodes and is positioned through guidance with ultrasound, according to the New York-based company said.
PAVmed won FDA 510(k) clearance for CarpX in April 2020.


![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)

