Prescient Surgical, a medical device innovator dedicated to developing advanced tools and technologies to prevent surgical site infections (SSIs), recently announced that the FDA has cleared the CleanCision Wound Retraction and Protection System (CleanCision System) for commercialization in the United States.
Developed by surgeons and infection control experts, CleanCision is the first in a new class of advanced technology designed to fight and defend against the most pervasive sources of surgical infection. Utilizing active cleansing technology, CleanCision combines wound protection and irrigation into an intuitive and easy-to-use retraction system that actively, consistently, and continuously clears harmful bacteria that may invade the incision during surgery.
Unlike traditional methods, which cannot continuously and consistently clear contamination from the surgical site, CleanCision has been shown to reverse and reduce these pervasive sources of infection, clearing harmful bacteria throughout surgery when the threat of wound contamination is at its highest.
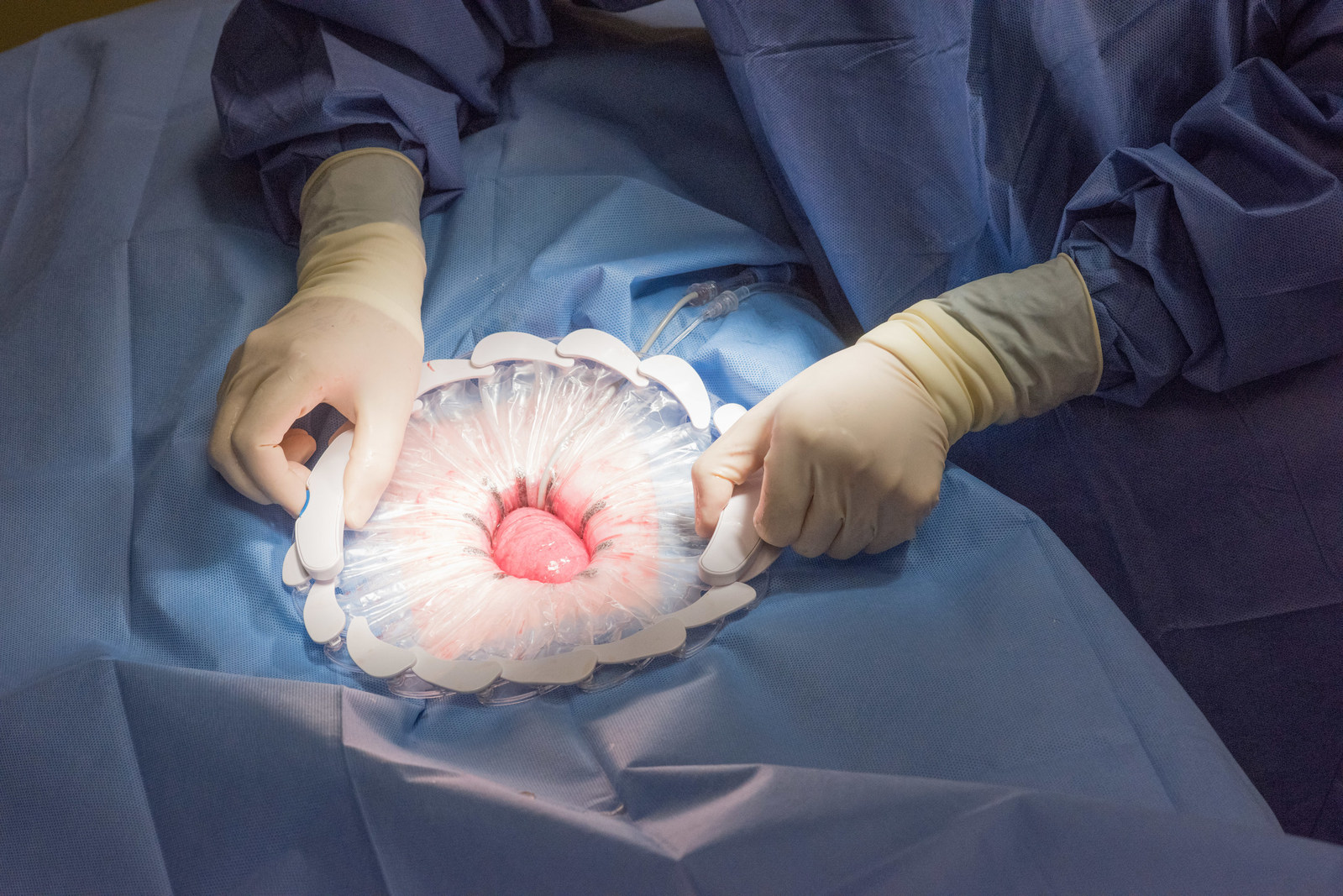
Developed by surgeons and infection control experts, CleanCision(TM) is the first in a new class of advanced technology, combining wound protection and irrigation into an easy-to-use retraction system that has been shown to reverse and reduce the pervasive sources of surgical infection, clearing harmful bacteria throughout surgery when the threat of wound contamination is at its highest. (Image credit: Prescient Surgical)
“The threat of incision infection in high risk abdominal surgery presents a constant threat with the potential to adversely impact patient outcomes and drive up health care costs for hospitals,” says Mark Welton, MD, chief medical officer, Fairview Health Services, former Harry A. Oberhleman Jr., professor of surgery and chief of colon and rectal surgery at Stanford University School of Medicine, and cofounder of Prescient Surgical.
“CleanCision can put more control over the root causes of infection into the hands of surgical teams, enhancing their current infection control protocols,” he adds.
“While infection control workflow and processes steadily improve year after year, the tools and technologies that should aid those efforts simply haven’t kept pace,” says Insoo Suh, MD, assistant professor of surgery, division of general surgery, endocrine surgery section, University of California San Francisco and attending, endocrine, and general surgery, San Francisco VA Medical Center, and cofounder of Prescient Surgical. “We are seeing that a proactive approach to clearing contamination during surgery has the potential to better protect patients from infection and help hospitals address the increased health care costs that result from surgical site infections, such as extended hospital stays, re-hospitalization and rising infection rates that trigger penalties from CMS.”
Easily deployed to retract and protect the wound site, CleanCision provides access to the surgical site and uses a sterile irrigant solution, selected by the surgeon, to clear contamination invading the surgical incision. The wound edge is continuously and consistently irrigated while suction removes contaminants throughout the surgery. CleanCision is cleared for use in abdominal surgery and may aid in the prevention of wound edge contamination.
“We are initially focusing on abdominal surgery and particularly colorectal surgery, where the risk, frequency and severity of surgical site infection is high and the need is acute,” says Jonathan Coe, cofounder, president and CEO of Prescient Surgical, Inc. “Our team collaborated closely with leading hospitals in abdominal surgery to create a technology platform and product that could be used in the full range of open and minimally invasive approaches utilized in their procedures. The result is an intuitive system that readily integrates into surgical workflow.”
Hospitals can acquire CleanCision through the company’s Early Access Program, developed to help surgical teams implement and measure the impact of the device as part of their infection reduction programs.




