The FDA’s Center for Devices & Radiological health granted de novo clearance for Prescient Surgical’s CleanCision Wound Retraction and Protection System for surgical wound edge protection, retraction, and continuous cleansing with a sterile irrigant solution. The CleanCision device may also aid in the prevention of wound edge contamination, a powerful advance towards mitigating associated infections.
The CleanCision device, the first in a new category of irrigating wound protection devices classified by the FDA, provides access to the surgical site while protecting the incision with an impermeable barrier, defending against direct contamination. The device also facilitates intraoperative delivery of a sterile irrigant solution of the surgeon’s choice to the wound edge. With CleanCision, wound irrigation and wound protection, two independently proven strategies to prevent surgical site infection, are seamlessly combined into a single device.
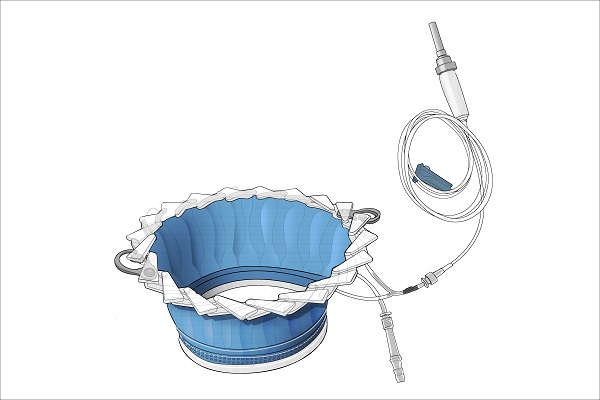
(Image credit: Prescient Surgical)
Prescient Surgical was spun out of the Stanford Byers Center for Biodesign in 2012 to address the risk of surgical site infection (SSI), a serious complication associated with significant morbidity and mortality. SSI risk is generally 2-5 percent in the 30 million surgeries conducted annually in the U.S., but can be as high as 15-50 percent in certain high-risk, clean-contaminated, and contaminated procedures like colorectal surgery. Recent changes brought about by the Affordable Care Act, including public reporting and financial penalties, have heightened national focus on SSI prevention.
“This news from the FDA marks a crucial milestone for Prescient Surgical and validates the hard work put in by our team and clinical partners towards our mission of reducing the risk of SSI. Most importantly, we can now look toward extending the promising results we’ve seen in recent clinical trials to the broader patient population,” Prescient Surgical CEO Jonathan Coe said in a statement.
The timing of the de novo clearance is fortuitous. According to Brant Heise, Managing Director at Summation Health Ventures, “Hospitals are increasingly focusing on infection control to improve patient care as a primary goal. Prescient’s success with the CleanCision device bodes well for providers driven towards the ‘triple aim’ of quality of care, patient satisfaction, and reduced costs.”
Prescient Surgical plans to launch the CleanCision device in 2017.




