Scientists of the Leibniz-Institute of Photonic Technologies (Leibniz-IPHT), Center for Sepsis Control and Care at the University Hospital Jena and Friedrich Schiller University work at a faster and cheaper alternative for hitherto time consuming pathogen diagnostics. Project manager Prof. Ute Neugebauer illustrates the advantages of this new approach: “We combine light-based analytical methods with microfluidic sample processing. With our Lab-on-a-Chip system, thus a miniaturised lab, we are able to clearly identify bacterial strains and their resistances, in less than three hours”.
Standard practices for the infectious diagnostics require up to 72 hours to allow for a reliable result. This is due to the fact, that the number of pathogens in a patients sample is too small to conduct tests. Analysis is therefore only possible after time-consuming cultivation. Especially in clinical application during treatments of severe infections e.g. a sepsis time is a crucial factor. Intensive physicians are confronted with an alarming dilemma: “far too often we have to administer broad-spectrum antibiotics ‘blindly’, because we can neither analyse pathogen nor potential resistances. Therefore, we possibly use a sledge-hammer to crack a nut. A vicious cycle that aides the development of new resistances”, explains Prof. Michael Bauer, director of the Clinic of Anesthesiology and Intensive Care at the University Hospital Jena.
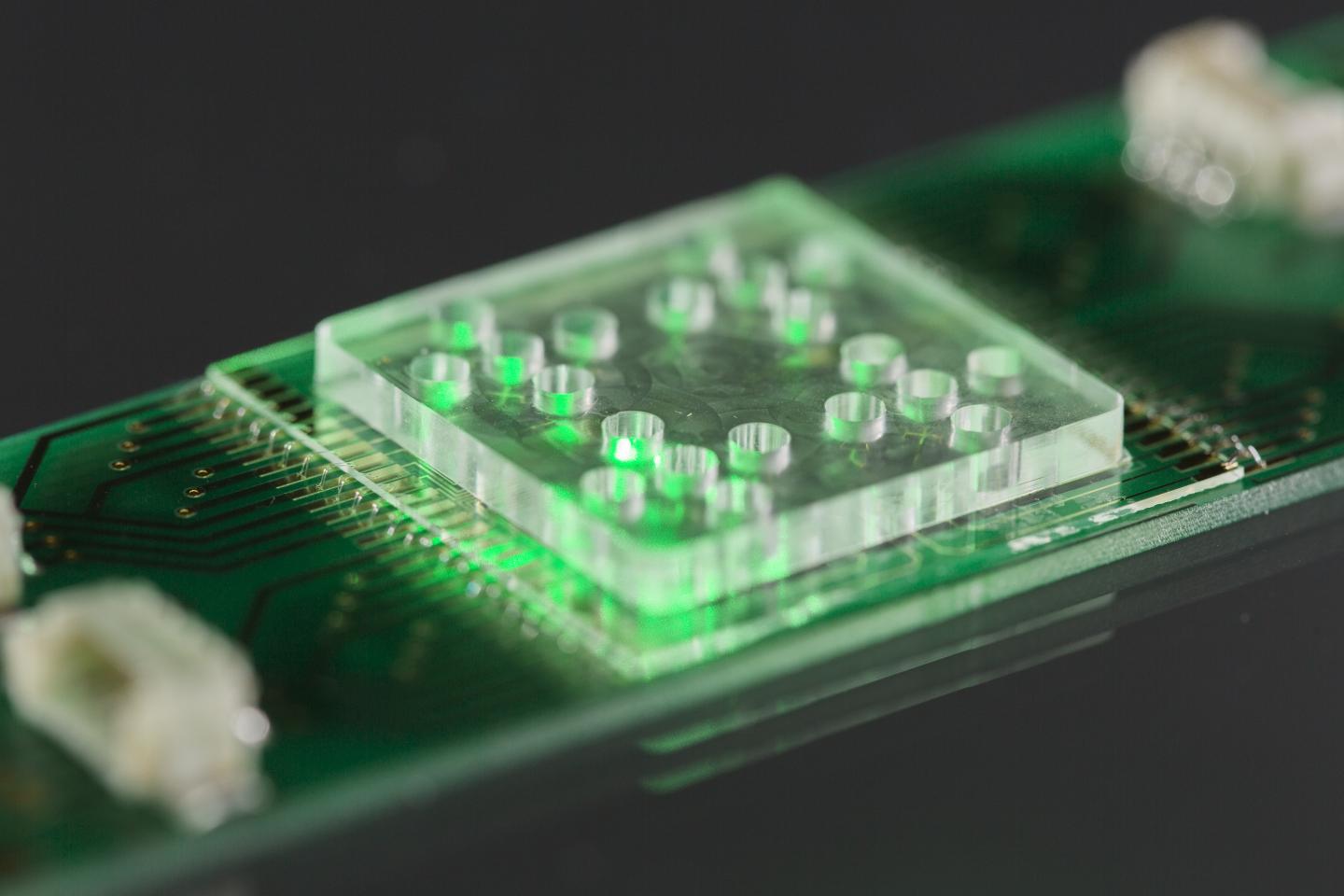
The new method out of Jena provides much faster diagnosis as basis for a decision of a reliable therapy. Ute Neugebauer, who works at Leibniz-IPHT and the University Hospital Jena points to tiny electrodes that are fixed on the surface of a stamp-sized chip: “Electric fields secure bacteria in a very small area”. Jena’s scientists then apply various antibiotics in different concentrations on the trapped bacteria and examine them with Raman spectroscopy. “This means that we irradiate the pathogens with laser light and evaluate the scattered light spectrum”, describes Neugebauer the method.
Prof. Jürgen Popp, director of the Leibniz-IPHT and head of the Institute of Physical Chemistry of the Friedrich-Schiller University Jena, explains: “After two hours we can already detect distinct changes in the Raman spectra. Out of these, we can derive wether the strain is resistant or sensible. At the same time we get information on the needed concentration of the antibiotic to constrain bacterial growth. This is an important diagnostic parameter that influences the success of a treatment decidedly”, Popp continues. The results of the team of chemists, physicians, and biologists were published in the current edition of the renowned journal Analytical Chemistry, which was released in February 2018.
The combination of fast, light-based diagnostics and a high automation level reduces the time from sampling to result from to date 72 to three and a half hours. “Such a fast procedure could revolutionise diagnostics of infectious diseases”, Prof. Bettina Löffler, director of the Institute of Medical Microbiology at the University Hospital Jena, is sure about that. Currently, researchers work at a platform for the application in hospitals. Another, more far reaching, aim is the further development into a catridge-based rapid test system, which will enable general practitioners to identify resistances in a fast and easy way for the first time. Thereby, physicians would hold a powerful tool, from which they could benefit in personalised therapy, this means the administration of a fitting drug.
CREDIT: S. DÖRING/ LEIBNIZ-IPHT




