PROCEPT BioRobotics, a robotics company developing surgical solutions to treat prostate disease, announced the completion of patient enrollment into the global Phase III WATER Study (Waterjet Ablation Therapy for Endoscopic Resection of prostate tissue).
The WATER Study is a double-blind, prospective, randomized clinical trial for male patients between the ages of 45 and 80 years old who have urinary symptoms due to benign prostatic hyperplasia (BPH). The Study, conducted under U.S. investigational device exemption (IDE), evaluates the safety and effectiveness of the AquaBeam® System as compared to transurethral resection of the prostate (TURP). The primary end points of safety and effectiveness will be measured at three and six months respectively, and patients will be followed out to three years to collect long-term clinical data.
(Credit: Procept BioRobotics)
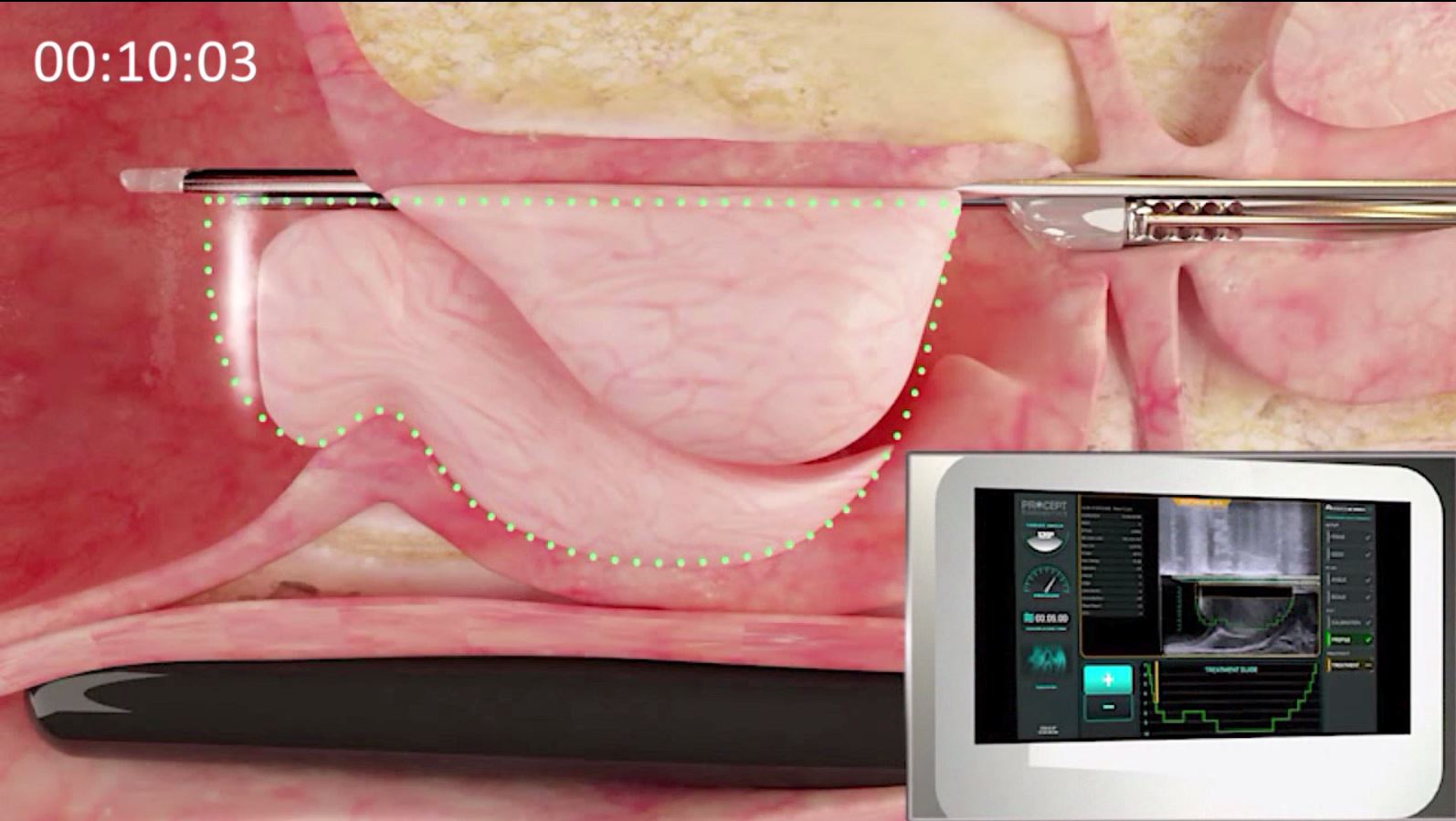
The AquaBeam System combines real-time prostate imaging and surgical robotics to deliver Aquablation, a waterjet ablation therapy that enables targeted, controlled, heat-free and immediate removal of prostate tissue for the treatment of lower urinary tract symptoms caused by BPH. Built-in simultaneous cystoscopic and intra-procedural ultrasound image guidance empowers the surgeon with improved decision making by enabling the surgeon to draw a treatment contour that conforms to the shape of the prostate adenoma while sparing the anatomical landmarks responsible for continence and ejaculatory function. The robotically controlled waterjet then precisely and efficiently resects the adenomatous tissue according to the prescribed treatment plan.
“The robust design of the WATER Study will provide us with comprehensive data related to the safety and effectiveness of Aquablation and a solid comparison to TURP, the most common procedure performed world-wide for the treatment of lower urinary tract symptoms due to BPH,” according to co-principal investigator Claus Roehrborn, MD, Chair of the Department of Urology at UT Southwestern in Dallas, Texas. “Thanks to the focus and commitment of the high-performing centers across three continents, we were able to complete enrollment of this ambitious study without delay.”

(Credit: Procept BioRobotics)
“By combining image guidance and robotics, the AquaBeam System has the potential to standardize BPH surgery through improved decision making and procedural predictability,” said co-principal investigator Peter Gilling, MD, Professor of Surgery at the University of Auckland, Tauranga Hospital, Tauranga, Bay of Plenty, New Zealand. “We were the first center to utilize this technology four years ago, and it has been exciting to witness the short learning curve at 16 other centers resulting in reproducible outcomes with this technology.”
The WATER Study successfully enrolled 184 patients at 17 sites across four countries. “It was a tremendous accomplishment to complete this rigorous clinical trial in one year, and I want to acknowledge each of the sites and their investment in clinical research,” said Nikolai Aljuri, Ph.D., co-founder and Chief Executive Officer of PROCEPT BioRobotics. “The completion of the WATER Study is an important step in achieving our goal of bettering the lives of men suffering from BPH, by developing a minimally invasive solution that offers both a sustained and significant improvement to quality of life and a reduced risk of sexual side effects.”




