Propeller Health, the leading digital solution for respiratory medicine, today announced U.S. Food and Drug Administration 510(k) clearance to market its Propeller platform for use with GSK’s Ellipta® inhaler, the pharmaceutical company’s innovative, patented, dry powder inhaler (DPI). The sensor for the Ellipta inhaler was built and cleared as part of a development agreement and R&D collaboration between Propeller and GSK that was announced on December 1, 2015.
“Today, we are pleased to announce the FDA clearance of the Propeller platform for use with GSK’s Ellipta inhaler,” said David Van Sickle, CEO and co-founder of Propeller. “Inclusion of GSK’s Ellipta inhaler in Propeller’s digitally-guided therapy platform is an important step in our goal of modernizing the management of respiratory disease. We look forward to working closely with GSK to deploy sensors for the Ellipta inhaler in the U.S. and abroad, in the near term.”
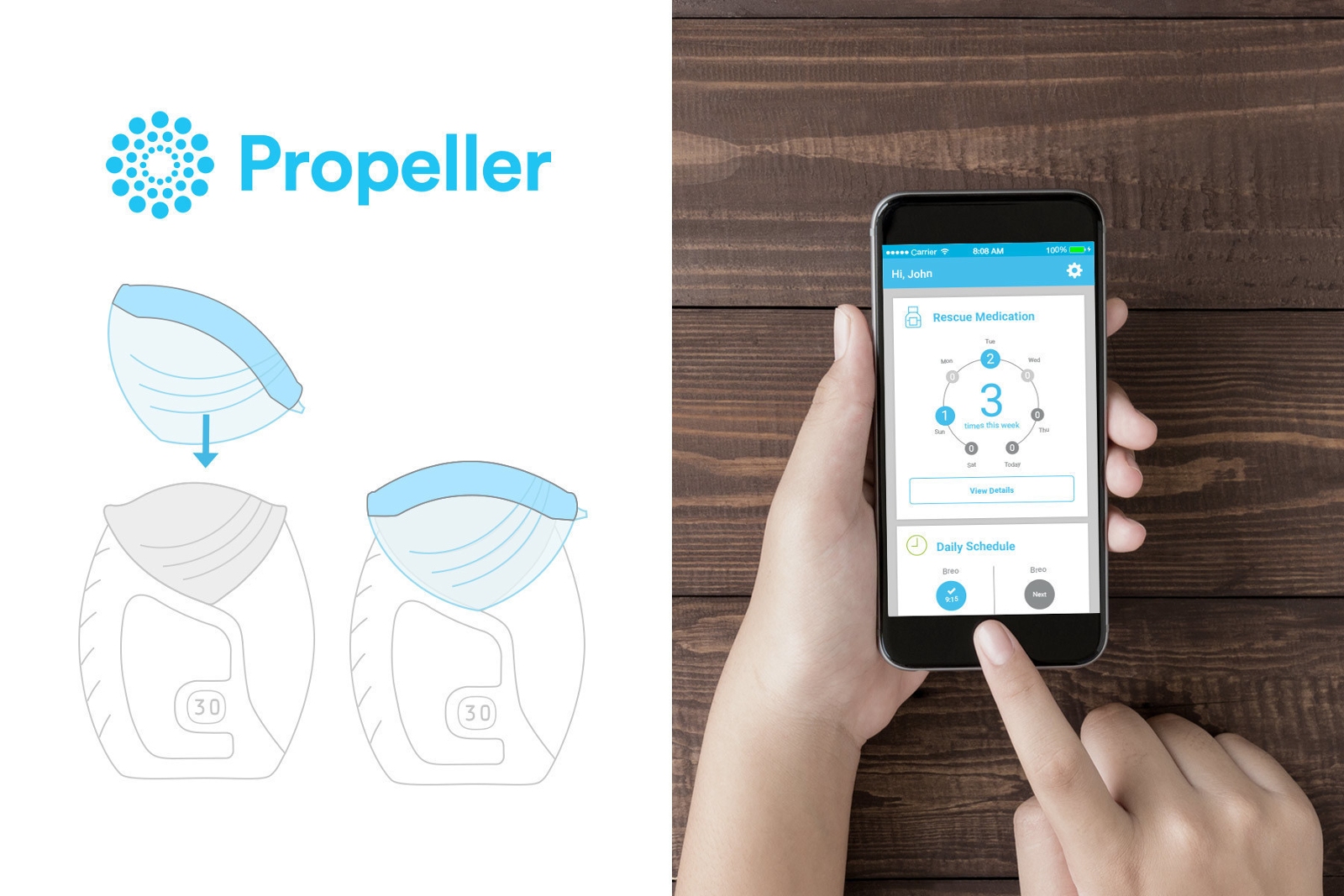
Propeller platform for use with GSK’s Ellipta(R) inhaler (Credit: PR Newswire)
This FDA clearance follows CE Mark and Health Canada registration for the device and system earlier this year.
Propeller is FDA-cleared to help patients and their physicians better understand asthma and COPD, and help to improve the symptoms and outcomes of these chronic respiratory diseases. With proprietary sensor technology, software, and services, Propeller’s digitally-guided therapy platform integrates information from multiple sources, including connected medications, then uses machine intelligence to help individuals manage their condition.
Dave Allen, head of respiratory R&D at GSK, said, “While it is still in the early stages of development, the emerging field of digital healthcare holds great promise for respiratory medicine. The approval of the Propeller platform for use with the Ellipta inhaler will help us understand how patients interact with the Ellipta inhaler accurately and in real-time. By exploring the benefits of sensor technology in this way, we hope to gain valuable insights into usage patterns with the ultimate goal of driving improvements in patient care while reducing the complexity and cost of clinical trials.”
The Propeller Health platform has been used by patients with asthma or COPD in over 45 commercial programs across the US, including major healthcare systems, payers, employers and other commercial partners. This clearance marks the eighth FDA clearance, as well as the eighth international clearance, received by Propeller to date.




