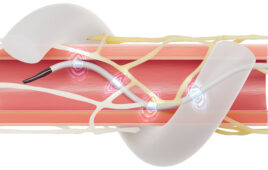
Proven Process Medical Devices, Inc. recently announced that its client Flowonix Medical Inc. has secured PMA-Supplemental approval from the FDA for its next-generation intrathecal infusion device, the Prometra II system. Approval of this device, created with the assistance of Proven Process’ design and manufacturing services, will enable Flowonix to proceed with product introduction in the coming months. The groundbreaking design for the Prometra II’s flow-activated valve was made possible in part by Proven Process’ Concept-to-Customer approach which helped Flowonix achieve important milestones and approvals for a successful product introduction.
The drug infusion device is novel in many ways, most significantly for its patented flow-activated safety valve (FAV), which allows patients to have an MRI without the necessity of drug removal prior to the procedure. MRI systems tend to emit significant electromagnetic energy that can interfere with operation of a patient’s implanted device. The Prometra II increases patient safety and clinical convenience by providing safe, dependable, automatic dosing of drugs directly into the intrathecal space around the spine. The Prometra II’s innovative FAV was designed to shut off drug flow to the patient in the event a high flow rate occurs during an MRI procedure.
With over 20 years of experience designing and manufacturing Class II and Class III medical devices for prominent global healthcare brands as well many start-up entities, Proven Process understood the technologies and best solutions to successfully create the small, self-contained, battery-powered device that delivers intrathecal pain therapy needed by over 100 million Americans suffering from chronic pain. The company’s vast experience ranges from implantable medical devices to complex cardiac devices, in-vitro diagnostic devices, disposables, robotics and sterile kits. Proven Process partnered with Flowonix to design and manufacture the newest addition to its brand of state-of-the-art Prometra drug infusion devices.
Proven Process’ Concept-to-Customer process guides medical device manufacturers seamlessly through the device development process, from a nascent idea to a feasible concept, then on to final design, verification, manufacturing and through the regulatory process.
Proven Process Medical Devices Inc.
www.provenprocess.com