Researchers at Purdue University have created a device that can quickly and inexpensively determine whether new pharmaceutical formulations have trace crystallinity that can negatively impact the drug’s stability and bioavailability.
The researchers have developed instrumentation that can accurately detect in early stages whether a pharmaceutical formulation has trace crystalline content. The instrument is based off of triboluminescence and works by measuring light emitted when a pharmaceutical powder is crushed.
“Any light that is measured is directly proportional to how much crystallinity is in the formulation,” said Casey Smith, a graduate student in Purdue’s Department of Chemistry.
The rest of the story can be found on Purdue University News.
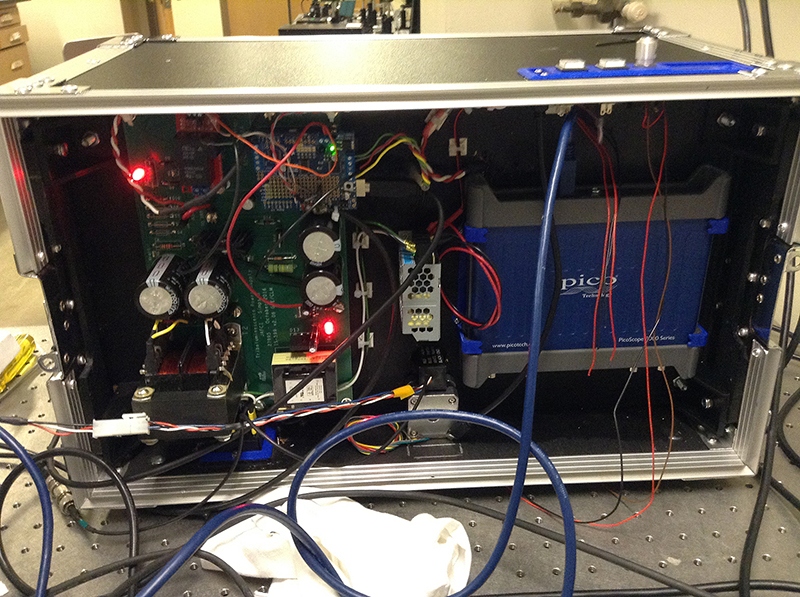
The back side of an instrument created by Purdue University researchers to determine if pharmaceutical formulations have trace crystallinity. The main circuit board that controls the instrument consists of a high-power voltage regulator to power the striking solenoid. The instrument was created in collaboration with the Department of Chemistry’s Jonathan Amy Instrumentation Facility.


