Although many design engineers rarely think about packaging, it may be time to take a fresh look at the stuff that holds, protects and presents the products they work so hard to create. The traditional role of packaging was to protect it against damage during transport and provide a sterile barrier against contamination. Recently however, innovative designers are asking packaging to take on new functional roles, acting as part of the delivery and preparation process.
Several conversations I had during my recent visit to the MD&M East conference have convinced me that including packaging as an integral part of a product’s overall design can result in improved convenience and functionality and, in many cases, reduced total solution cost. In addition, it can eliminate many of the costly last-hour panic situations which often occur when packaging is treated as an afterthought. “Co-designed packaging requires close coordination between the product design team and the packaging manufacturer,” said Lynne Barton, Senior Account Executive at SencorpWhite, “but the added effort pays off in terms of improved functionality, customer satisfaction, and total solution cost.”
Those conversations also revealed that achieving these benefits will involve taking a new approach to the design process. “Co-designing a medical device and its packaging begins with understanding end needs for the device at time of use”, said Aneta Clark, Market Development Manager of Eastman Chemical Corp’s medical packaging division.
Clark also told me that an effective co-design requires a good working knowledge of the materials available for the packaging solution. She explained to me that choosing the right packaging material is influenced by a surprising number of factors, including how the product will be sterilized, processed and prepped for use are primary considerations, as well as the temperature range it will experience during storage and use. A good working knowledge of material properties also helps designers exploit a plastic’s properties to produce the unique structural and functional features (undercuts, hinges fastenings, etc…) that enable a device’s package to become a valuable element of the total product solution.
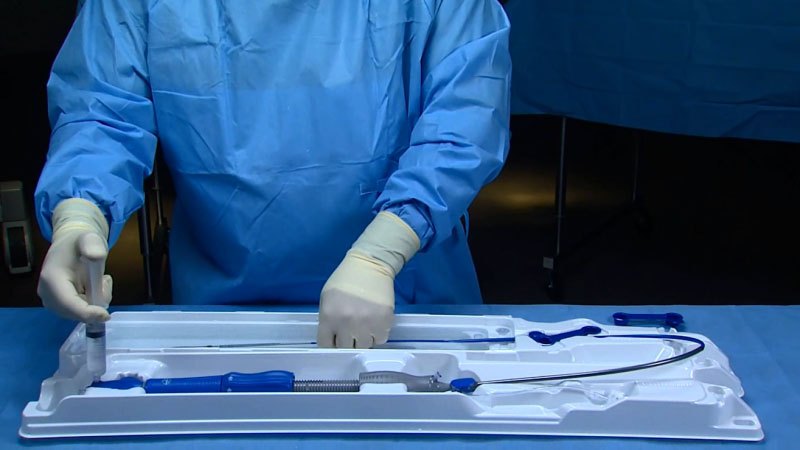
Photo courtesy of Medtronic.
As an example, Clark cited Medtronic’s EnVeo R, a next-generation Transcatheter Aortic Valve Implantation (TAVI) system that’s used to treat sick patients who otherwise would not receive valve replacement therapy through surgical means. The system’s mission is to deliver a porcine tissue heart valve via a catheter delivery system using a minimally invasive technique. Thanks to close consultation with the surgeons and OR personnel who would be using the EnVeo R system, both the transcatheter device and the packaging that houses it incorporate several innovative design features. For example, the TAVI is capable of recapturing the valve prior to full release, making it easier for surgeons to assure precisely correct placement.
The system’s packaging also contains several unique capabilities of its own, including a folded configuration which gives a single user complete control of the 62-in.-long catheter while it’s still being held securely in its sterile tray. This greatly simplifies the preparation and loading of the valve onto the catheter. The tray also includes wells which hold all the accessory parts associated with the TAVI as well as areas specifically designed for rinsing and preparing them for the procedure. The design team also incorporated visual aids into the tray which assist in loading the valve onto the catheter. As a result of the co-design process, the TAVI occupies less precious OR space and requires fewer nurses to set up the valve for insertion.
Do you have any packaging success stories that you’d like to share with your fellow MDT readers? Write me at: Lee.Goldberg@AdvantageMedia.com.




