ZipLine Medical, Inc. an innovator in skin closure, recently announced publication of positive results from the first prospective, randomized, controlled study of its Zip Surgical Skin Closure in pediatric surgery. In the study, the Zip demonstrated significant improvements in scar appearance, efficiency, and patient pain compared to suturing. Results were published online in the Annals of Thoracic Surgery.
The study of 214 pediatric patients undergoing cardiovascular surgery compared the Zip to continuous subcuticular polypropylene sutures for skin closure at three months. The study found:
- Significant improvement in scar appearance with the Zip, as evaluated by two plastic surgeons using the Vancouver Scar Scale (VSS) (p<0.017)
- Three times shorter skin closure time with the Zip (113.0 ± 9.1 seconds versus 375.9 ± 60.2 seconds, p<0.001)
- Significantly fewer patients cried or complained during removal of the Zip (7.1 percent versus 52.5 percent, p<0.001)
The article is entitled, “Randomized Study of a New Noninvasive Skin Closure Device for Use After Congenital Heart Operations.” The study was conducted at Gunma Children’s Medical Center, Shibukawa, and the Department of Cardiovascular Surgery, Kitasato University Hospital, Sagamihara, both in Japan
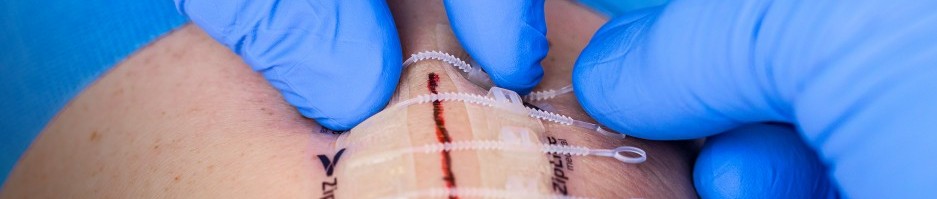
(Image credit: ZipLine Medical)
The authors conclude, “Our study showed that the [Zip] device has an excellent performance in improving the cosmetic appearance and reducing skin closure times.” They add, “Use of this device was not affected by the length of the wound or by different surgeons,” and “the tension can be adjusted multiple times according to the extent of the edema or wound healing after the operation.”
The Zip is a non-invasive and easy to use device providing secure wound closure with uniformly distributed force along the length of the incision. Clinicians can adjust closure tension at any time peri- or post-procedure. Because closure and removal are simple, clinicians can delegate the task to a physician assistant or nurse, and patients may remove the Zip at home, if appropriate. The flexible design is not only comfortable for patients, but often enables them to make quicker progress in their recovery. Because there are no skin punctures with the Zip, scarring is minimal and there are no added channels for infection to enter.




