Technological innovations in the life sciences are making life better for the chronically ill. Hope, for these individuals, often lies in something as small as a human cell. Cellular therapies offering regenerative abilities are growing this hope within patients of all ages — and in their doctors. But as healthcare services among those with diabetes, cancer, heart disease, and neurological diseases expand, clinical, diagnostic, and pharmacological laboratories will have to increase their investment in automation.
Enabling technologies like high resolution optics, precision modular automation, precision fluid handling, and quantitative image analysis embedded in an ecosystem optimized for cells and cell technicians are being incorporated into instruments, giving researchers a greater ability to qualify stem/progenitor cells and to manipulate key colonies of interest.
James Price, the president and chief executive of the Canadian Stem Cell Foundation, said, “Stem cell therapy will transform healthcare and how we treat devastating diseases. We are on the verge of breakthroughs that will fundamentally alter the treatment of illnesses such as diabetes, cancer, heart disease, and neurological diseases.”1
Analyzing Cells with Accuracy
The problem that many researchers in this field have faced is the inability to use large field of view (LFOV), high resolution optics to image cell cultures to monitor and quantify cell-based changes. Additionally, many devices lack the accuracy and precision required for rigorous sampling, transfer, or deletion of specific cells or colonies.

Precision automation in the Cell X Imaging Analysis and Picking System is making it possible to perform quantitative cellular analysis.
Today, most cell-based research and cell fabrication strategies are supported by manual processes. Colonies are often visually assessed and identified, counted or selected, and manually transferred by a lab technician working with a microscope, viewing a sample through a narrow field of view. Given the mundane and highly subjective nature of this method, results are highly inconsistent and subject to human error.
Quantitative methods for cell and colony metrics are typically limited to destructive assays, in which groups of cells are combined and analysis of the proteome or gene expression of the entire population is assessed. Although these destructive methods provide accurate quantitative information, they only provide an average assessment of the cell colony type. An automated image-based system would allow for both accurate quantitative metrics of biological performance (cell size, number, morphology, surface markers, extracellular matrix properties, and — in some settings — gene expression or secretory profile). Moreover, these metrics can be applied on a cell-by-cell or colony-by-colony level, as well as at the level of gross population metrics.
In contrast, a manual colony count, the most common method for quantifying the number of stem cells or progenitors in a given sample, is highly variable. Manual counts of the same sample frequently differ by 100% between observers and within the same observer at different times. Furthermore, beyond just a number of colonies, manual counts fail to provide quantitative data on the features of each colony that was counted. Nor do such counts create a durable record of the status of each colony at the time of assay.
Despite the enormous progress and promise in the field of cell therapy development, unresolved challenges continue to plague the community exploring cell-based diagnostics. Dr. George Muschler of the Cleveland Clinic, the Cell X innovator, characterizes these unresolved issues, “A critical gap in current stem cell and progenitor cell biology that limits translation into effective therapies is our current inability to define and measure critical quality attributes in the stem cell and progenitor populations in our labs today. These attributes must be defined based on quantitative and standardized metrics. With this information, we can then act, using appropriate tools, to rapidly identify and isolate desired cells or clones, or to delete undesired cells/clones from our clinical therapies and critical research studies.”

An mSR Series positioner from Parker positions cells for imaging and manipulation.
Automated Cell Handling
Cells are tiny, living, breathing things; as such, they must be handled with precision and care. Automated handling equipment in particular needs a heightened level of dexterity and control. When looking at typical therapy processes, there are effectively two major processes.
- Scanning and identification — Using optics, in conjunction with motion systems and control algorithms, to find cells or clusters of cells of interest and identify their location in 3D space.
- Cell selection process — Utilizing interactive surgical tools to selectively biopsy cells of interest, remove undesired cells, or transplant desired cells into a site where they can be further expanded, processed, or studied.
Parker Hannifin has designed several different platform products for manufacturability, modularity, and most importantly, performance. The scanning and identification process directly aligns with its experience in digital pathology, enabling high precision, on-the-fly capture of high resolution images of cell biopsies. The cell selection process directly aligns with the company’s experience in additive manufacturing, precisely placing material where it belongs.
Parker worked with Dr. Muschler and his team, including medical institutions and Biospherix, provider of the Cytocentric platform, to develop the Cell X device. Cell X combines LFOV imaging and control capabilities with precision instrumentation, fluidics, and documentation. Cell X opens new dimensions of opportunity for discovery and development of stem cell therapeutics. These same features also provide new avenues for quantitative personalized cell-based diagnostics, optimization of biomaterials and bioactive surface design, and drug discovery applications.
The central core of the device is a high quality automated inverted microscope and CCD camera, with both brightfield and fluorescent imaging options.
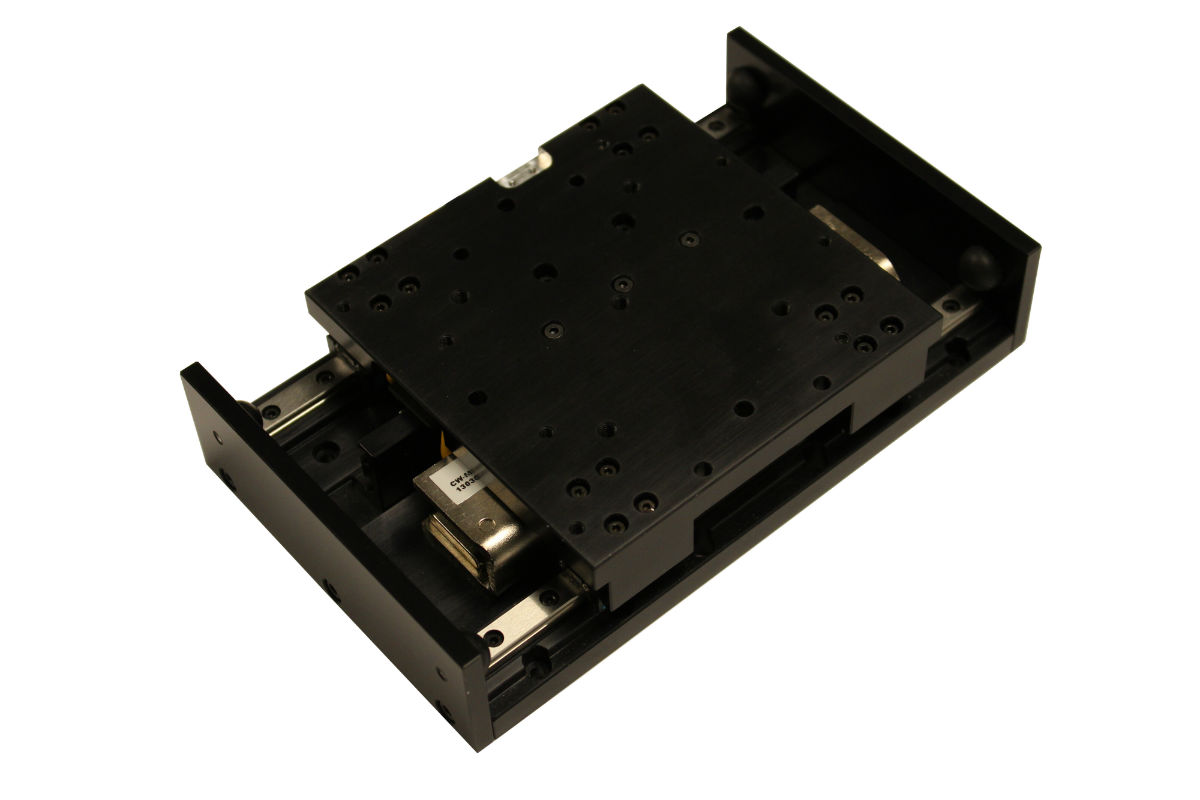
Parker’s mSR Series compact linear positioners combine smooth motion with sub-micron level precision, for ±0.1 μm repeatability.
Other enabling features added to this standard laboratory equipment are:
- High precision low profile XY system — This positions the cell carrying trays in the optical system with submicron repeatability (0.1µm). Integrated high resolution feedback devices allow the robot to precisely determine the exact position of cells. Custom trays allow the use of any standard cell culture plate or dish, as well as options for automatic loading and unloading of plates. The low profile of this design was key for keeping optics in the focal range of the microplates.
- Rigid machine base — This base was custom engineered to align the microscope and positioning axes. This structure provides a very stiff, stable platform with integrated isolating pads to minimize the vibration the robot might see from external sources.
- Light filter adjustment axis — A high precision miniature ballscrew-driven axis used to adjust filters automatically to optimize the lighting of the cells.
- Tip load and remove station — Disposable tips used in picking cells of interest are automatically loaded and removed without human intervention.
- Z-axis positioners — Three Z axes enable the picking and placing of cells or fluids. The first axis uses Parker’s Smart Syringe pump to aspirate the cells of interest. The second axis is used for precise media removal around the area of interest. The third axis allows for additional steps for multiple fluids or reagents.
- Cell optimized ecosystem and cell technician optimized work space — Cytocentric platform complements machine by neutralizing typical negative side effects of machine on cells (particles, heat, vibration, etc.), and synergizes with machine by optimizing all the critical cell process parameters (temperature, humidity, CO2, O2) continuously upstream, instream, and downstream of machine. Cells are protected from contamination by ultra-aseptic conditions (zero CFU). Machine precision is protected from degradation by elimination of dust and temperature fluctuations.
Cell technicians have convenient, comfortable access to the machine and all cell process steps before and after. At the same time, they are fully protected from cells harboring dangerous viruses, vectors, prion, and other pathogens by 100% containment of all spills, splashes, and aerosols, thereby addressing critical biosafety concerns of cell technicians and associated liability.
Validation packages are available for GMP and other regulated applications. Unlimited expandability with compatible interconnecting modules is available for adding equally optimized manual or automated steps upstream or downstream of Cell X. Optimizing by simulating physiologic or pathophysiologic conditions generates the most relevant data and keeps phenotypic integrity intact longer. Preventing fluctuating and suboptimal conditions increases resolution of cell data and maximizes reproducibility of cell behavior.
“Only by attending to the holistic needs of cells and cell technicians, with a total quality approach, is it possible to realize the full potential of such a sophisticated machine,” says Randy Yerden, CEO of BioSpherix.
Integrated tip locating sensor — Disposable tips used in the cell selection process are not precision machined. To locate each new tip accurately, an integrated optical sensor was developed for finding each new tip’s location.
High performance motion controller — Functioning as the brains of this robot, a high performance motion controller is used to coordinate the motion between all axes and motion for fault conditioning.
The combined precision and imaging features in this cytocentric system have enabled leading researchers to collect data rapidly. Images taken from this system are of high enough quality and can be digitally stitched together to provide LFOV analysis and monitoring of colonies and cell populations. Due to the high repeatability of this system, each cell or colony can be given a digital address and revisited by the motion system at a later date to monitor time dependent changes.
What’s Next?
The future of cell therapy development involves having quantitative and reproducible standards that enable the determination and management of critical quality attributes for cells and colonies, in order to provide optimal safety and efficacy.
Meanwhile, technology will continue to develop in modularity, scalability, and precision to help researchers, practitioners, and their suppliers continue to improve the reproducibility of cell-based experiments, thereby accelerating the development of life changing cellular therapies.
References
1Elizabeth Payne, “Stem cell therapy advocates launch 10-year action plan (with video),” Ottawa Citizen, October 29, 2014, accessed August 28, 2015; http://ottawacitizen.com/news/national/stem-cell-advocates-therapy-launch-10-year-1-5b-action-plan.




