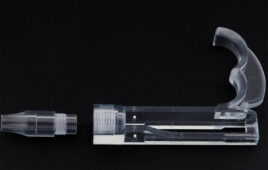
Roland DGA Corporation, a leading provider of digital fabrication tools including 3D milling machines, 3D printers and engraving machines, has announced the launch of a new UDI (Unique Device Identification) barcode impact printer, the METAZA MPX-95, as well as an optional DPM (Direct Part Marking) Kit, as an exclusive solution supporting medical and industrial instrument traceability.
Roland DGA Corporation, a leading provider of digital fabrication tools including 3D milling machines, 3D printers and engraving machines, has announced the launch of a new UDI (Unique Device Identification) barcode impact printer, the METAZA MPX-95, as well as an optional DPM (Direct Part Marking) Kit, as an exclusive solution supporting medical and industrial instrument traceability.
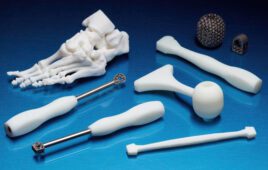
The MPX-95 with DPM Kit was developed for use in medical environments for permanently marking scalpels, forceps and other medical instruments, and in industrial settings for imprinting tools and equipment with unique identifiers. With its diamond stylus and powerful impact force, the MPX-95 can imprint on a wide variety of metals, such as stainless steel, iron, titanium, platinum gold, silver, copper, nickel and aluminum, quickly and with pinpoint accuracy. Imprinting individual medical instruments with a serial number, 2D symbol or other markings enables management of those instruments, including stock availability, location, usage history, and maintenance records.
According to Roland DGA’s 3D solutions product manager, Matt Anderson, regulatory bodies worldwide are seeking a comprehensive medical instrument management system. “They are looking for a way to manage these instruments effectively to reduce the risk of infection and better ensure patient safety,” Anderson explained. “The U.S. began phasing in the FDA Unique Device Identification regulation in September of 2014 requiring all medical devices to display a unique identifier. Within a few years, we expect similar regulation to become compulsory in the EU and Asian countries as well.”
The Roland MPX-95 quickly creates 2D DataMatrix barcodes to GS1*1 standards, which provide a common foundation for global business by uniquely identifying, accurately capturing and automatically sharing vital information about products, locations, assets and more. GS1 identification includes standards that define unique identification codes that can be used by an information system to identify and obtain information about instruments, tools and other physical objects.
The MPX-95 is capable of imprinting 2D DataMatrix barcodes on the surface of medical instruments in areas as small as one square millimeter, using dot pin marking to permanently mark surfaces and prevent erasure and corrosion. A removable base table and plate enables virtually all types and sizes of medical instruments to be marked with identifiers. The MPX-95 also comes standard with a laser pointer that assists in identifying the area to be marked. This feature enables even novice users, who may not be accustomed to using digital technology, to apply marking easily and accurately without any special training.
Roland’s DPM Kit, created for use with the MPX-95, comes with METAZA Studio software that makes it easy to generate a unique identifier for each instrument. The kit also includes an adjustable center vise with clamp pins for repeat personalization of irregular items, plus a DPM vise with multiple tooling options designed to mark instruments and equipment with contrasting dimensions in quick succession.
The Roland MPX-95 photo impact printer base unit has an MSRP of $4,995, while the optional DPM Kit is priced at $4,495 (MSRP).
To learn more about the new METAZA MPX-95, or the complete line of Roland products, visit www.rolanddga.com.
*1 The GS1 DataMatrix is a standard for 2D symbols determined by the GS1 international body for barcodes, symbols and electronic data transfer. It provides electronics manufacturers and medical facilities worldwide a standardized method to display symbols on instruments. Information on item code, usage limits, lot number, serial number and more can be stored within 26 bytes and read using a special scanner.
Roland DGA
rolanddga.com