Rotation Medical Inc., a medical device company focused on developing new technologies to treat rotator cuff disease, announced results of a biopsy study published online in Arthroscopy that supports the biocompatibility of the company’s bioinductive implant and its ability to promote new connective tissue with the histological appearance of tendon. The Rotation Medical bioinductive implant is part of the company’s novel rotator cuff system for rotator cuff repair and a new alternative to traditional surgical repair.
When the procedure is performed with the bioinductive implant in lieu of standard repair, the patient’s recovery is approximately two weeks in a sling and four or five sessions of physical therapy compared with the standard of care, requiring four to six weeks in a sling followed by four to six months of physical therapy. (Credit: Rotation Medical Inc.)
“This is a breakthrough study, as it is the first to show that the Rotation Medical bioinductive implant promotes the rapid growth of tendon-like tissue in humans,” said Dr. Steven P. Arnoczky, lead investigator of the study and director of the Laboratory for Comparative Orthopaedic Research at Michigan State University. “While clinical studies have demonstrated evidence of new tissue generation via imaging, the histologic character of this tissue could only be inferred. The unique opportunity to evaluate biopsies from implant recipients confirms the findings of the preclinical animal study, and demonstrates that the implant is biocompatible and promotes new connective tissue with the histological appearance of tendon over the surface of the native cuff tendon.”
The retrospective study included biopsies from seven patients undergoing a second arthroscopic procedure for issues unrelated to the implant at various time points (five weeks to six months) following arthroscopic rotator cuff repair with the Rotation Medical bioinductive implant. The biopsy specimens were examined histologically for host-tissue ingrowth, host-tissue maturation and host-implant biocompatibility. Key observations include:
- Five weeks: Presence of host cells (fibroblasts) within the interstices of the porous collagen implant. Cells were aligned along the linear orientation of the collagen implant structure, and there was evidence of early collagen formation.
- Three months: Increased collagen formation, maturation and organization over the surface of the implant and evidence of the collagen implant.
- Six months: Newly generated tissue with histologic appearance of a tendon, suggesting functional loading of the new generated host tissue. No evidence of any remnants of the collagen implant.
- No evidence of any adverse inflammatory or foreign body reaction at any time point.
“These biopsies underscore the safety of our Rotation Medical bioinductive implant in humans, as well as its ability to promote new tissue formation in a consistent and predictable manner,” said Martha Shadan, president and CEO of Rotation Medical. “We are encouraged by the results of this study, which provides further evidence that our bioinductive implant can biologically augment torn tendons and potentially reduce re-tear and revision rates.”
The study, “Histologic Evaluation of Biopsy Specimens Obtained After Rotator Cuff Repair Augmented With a Highly Porous Collagen Implant,” adds to the growing body of literature supporting the use of the Rotation Medical bioinductive implant as a novel treatment for rotator cuff injury. Additional publications and information about the Rotation Medical rotator cuff system are available on the company’s website.
About Rotator Cuff Injury and the Rotation Medical Rotator Cuff System
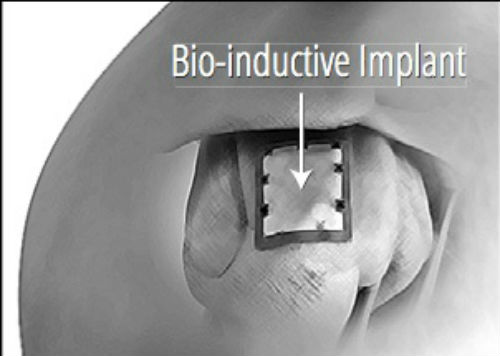
Rotator cuff damage is the most common source of shoulder pain, affecting more than 4 million people annually in the U.S. Traditional approaches to treating degenerated or torn rotator cuffs often do not address the poor quality of the underlying tendon tissue, and a significant number of these tendons, after standard treatment, either degenerate further and/or re-tear. Cleared by the U.S. Food and Drug Administration in March 2014, the Rotation Medical bioinductive implant is designed to address this limitation by inducing new tissue growth at the site of implantation, resulting in increased tendon thickness and healing of tendon defects with new tissue growth. The collagen-based implant is about the size of a postage stamp and it is part of the Rotation Medical rotator cuff system, which also includes disposable instruments that allow the arthroscopic procedure to be performed easily and quickly.
About Rotation Medical
Rotation Medical Inc. was founded in 2009 and is committed to improving the treatment of rotator cuff disease with the Rotation Medical rotator cuff system, a breakthrough technology that has the potential to prevent rotator cuff disease progression and reduce re-tears by inducing the growth of new tendinous tissue. The company is privately held and funded by New Enterprise Associates (NEA), Life Sciences Partners (LSP) and Pappas Ventures. For more information, visit www.rotationmedical.com.




