
The Scopio X100 (left) and new X100HT [Image courtesy of Scopio Labs]
The Tel Aviv-based digital microscope developer said the technology eliminates the need for manual microscopic examination when reviewing white and red blood cells and estimating platelet counts.
Based on the X100 that was 510(k)-cleared by the FDA in 2020, the X100HT has a slide loader with three cassettes that can each hold 10 slides. After the slides are placed in the cassettes and inserted into the slide loader, the slide loader automatically applies mounting media and a glass cover slip onto the slides and sequentially inserts them into the scanner for processing, according to the FDA’s decision summary.
The Full-Field peripheral blood smear (PBS) application technology on both the X100 and X100HT automatically locates, annotates and presents images of blood cells, flagging anomalies and suggesting classifications for review and confirmation. The user can evaluate red cell morphology, reviewing detected platelets and suggested platelet count estimates.
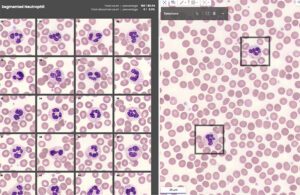
The Scopio X100HT’s AI-powered technology scans and annotates blood samples. [Image courtesy of Scopio Labs]
Scopio said the system can hold 30 slides and process up to 40 samples per hour, capturing large scan areas at 100x magnification. The results are automatically documented in a standardized digital report and easily shared, the company said.
“We’re excited to expand our suite of fully digital AI-powered diagnostic platforms to accelerate PBS analysis, improve consistency of results, and reduce review time,” Scopio Labs co-founder and CTO Erez Naaman said in the news release. “At Scopio, we are determined to usher in the digital revolution to laboratory medicine. Our devices offer complete remote capabilities for real-time diagnosis and treatment decisions, allowing experts to review, collaborate, and consult from anywhere, and at any time, using our AI-powered applications.”
Scopio is also developing an AI tool for enabling a complete digital workflow for bone marrow aspirates review, the company said.

![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)


