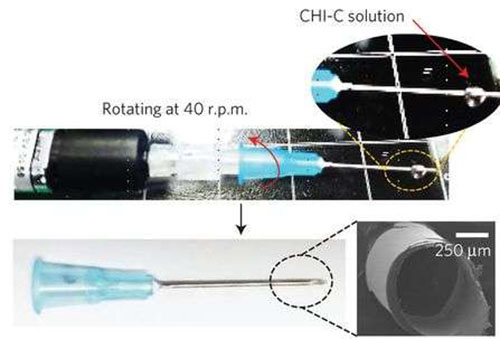
Preparation of a needle with self-sealing puncture properties and images of the needle coated with CHI-C film (SEM image). (Credit: (c) Nature Materials (2016)).
For people whose blood does not clot appropriately, such as those with hemophilia, diabetes, or cancer, getting an injection or blood draw with a hypodermic needle is not a trivial matter. Because the needle punctures the patient, this can result in continuous bleeding and risk of blood-borne infection. While researchers have looked at alternative methods, syringe injections are still the best method for rapid drug delivery. One solution could be a self-sealing syringe.
Researchers hailing from several institutions in the Republic of Korea have demonstrated that a syringe coated with a partially crosslinked catechol-functionalized chitosan thin film can successfully seal a puncture wound in mice. Furthermore, in mice and rabbits with hemophilia, their preliminary tests showed a 100% survival rate after injection. Their work appears in Nature Materials.
Shin et al. wanted a self-sealing coating for a hypodermic syringe that would not delaminate upon injection, but would upon removal of the syringe so that it would seal the puncture site. Inspired by the water-resistant secretions of mussels, they developed a catechol-functionalized chitosan polymer that can change from a thin film to a gel-like material when it comes in contact with blood plasma. They were able to make a homogeneous coating on syringe needles of varying gauges.
To test their concept, Shin et al. evaluated the technique with three different sets of mice. The first set of mice was punctured in the saphenous vein using a coated syringe in which the polymer had been pre-incubated for three days. The second set of mice was injected with a syringe without coating, and the third set of mice was injected with a syringe with coating that was not pre-incubated.
The mice that were injected with the pre-incubated syringe demonstrated no blood loss, while the bare syringe demonstrated the most blood loss. The non-incubated syringe evidenced delamination upon entry.
The incubation period results in the oxidation of some of the catechol (approx. 5.4%) to quinone, which serves to strengthen the polymer coating. In vitro viscosity tests showed that the non-incubated polymer did not change from a solid film to a gel-like material, but converted to a sol-phase, which resulted in film tearing. The incubated polymer underwent enough partial oxidation that upon exposure to human plasma, it converted to a more mechanically robust gel.
Their rabbit studies used a needle gauge of 23 G, which is the upper end of the size range used in humans. For these studies, Shin et al. looked at blood vessel morphology and evidence of polymer on the blood vessels. They also looked at coagulation time and changes in blood factor levels. They found that there was no appreciable blood loss in the rabbits tested and the hemostatic needle did not affect blood coagulation factors. Complete blood count was used to make sure that there was no evidence of reaction to foreign material.
These two tests provide a proof-of-principle for the self-sealing abilities of a hemostatic needle. The next step was to test mice with hemophilia. Hemophilic mice were injected in the jugular vein using either the polymer-coated hemostatic needle or a bare needle. All of the hemophilic mice that were injected with the hemostatic needle survived while the others did not. Shin et al. obtained similar results with other injection sites (e.g., intermuscular injections).
This promising research opens the door for a way that those with anticoagulating diseases can have the advantages offered by syringe injection and blood draw without the risks associated with an open wound. Future research could involve using this polymer coating in other medical devices.




