Fueled by rapid advancements in consumer biometric sensor technology, the mass proliferation of smartphones, and the increasing adoption of IoT products, medical-grade consumer wearables are now becoming a reality. While it is true that the best-selling wrist wearable brands have been publicly ridiculed by clinical professionals for poor sensor accuracy and misleading claims, the marketplace has seen the rise of consumer medical startups that have developed and clinically validated accurate, medically relevant technologies suitable for regulatory approval. In a sense, the marketplace is moving from “mood ring” to “medically relevant”.
The two biometric sensor modalities primarily used in medical-grade consumer wearables are based on mature technologies: Electrocardiograph (ECG), the measurement of the heart’s electrical activity, and photoplethysmography (PPG), the optical measurement of a change in volume in blood vessels due to heart beats. However, significant advancements were required to make these dated technologies wearable in a mobile environment. ECG had a major head start, while PPG sensor technology has only recently become “good enough” for medical use cases.
For ECGs, the development of robust, dry electrode technology was critical for eliminating the need for consumers to apply sticky gels or water to sensor electrodes. For PPG technology, the development of motion/environmental-tolerance was key. Before such development, PPG was rendered useless for wearables, as optical signals were overwhelmed by noise from motion artifacts and ambient lighting. Pulse oximeters, based upon PPG technology, were limited to resting or non-active use cases. To overcome these limitations, innovations in both wearable light-guiding and signal processing were required to enable sufficient tolerance to motion/ environmental noise.
When I first tested the best-selling medical pulse oximeter outdoors back in 2009, I was surprised find out that the oximeter simply did not work outdoors. This probably should not have been a surprise since the technology was originally developed for clinics and hospitals, not for ambulatory or lifestyle use.
When comparing ECG and PPG in context of medical wearables, PPG generally tends to have more pros than cons. Table 1 below summarizes the strengths (green) and weaknesses (red) of each sensor modality for various relevant attributes. It can be seen that form factor diversity and biometric diversity are where PPG shines through. Although PPG is less power efficient than ECG, mostly due to the need for powering their optical emitters, the technology has enjoyed substantial improvements in efficiency so that today’s sensors consume about 1 percent of the power a similar device would use only eight years ago.
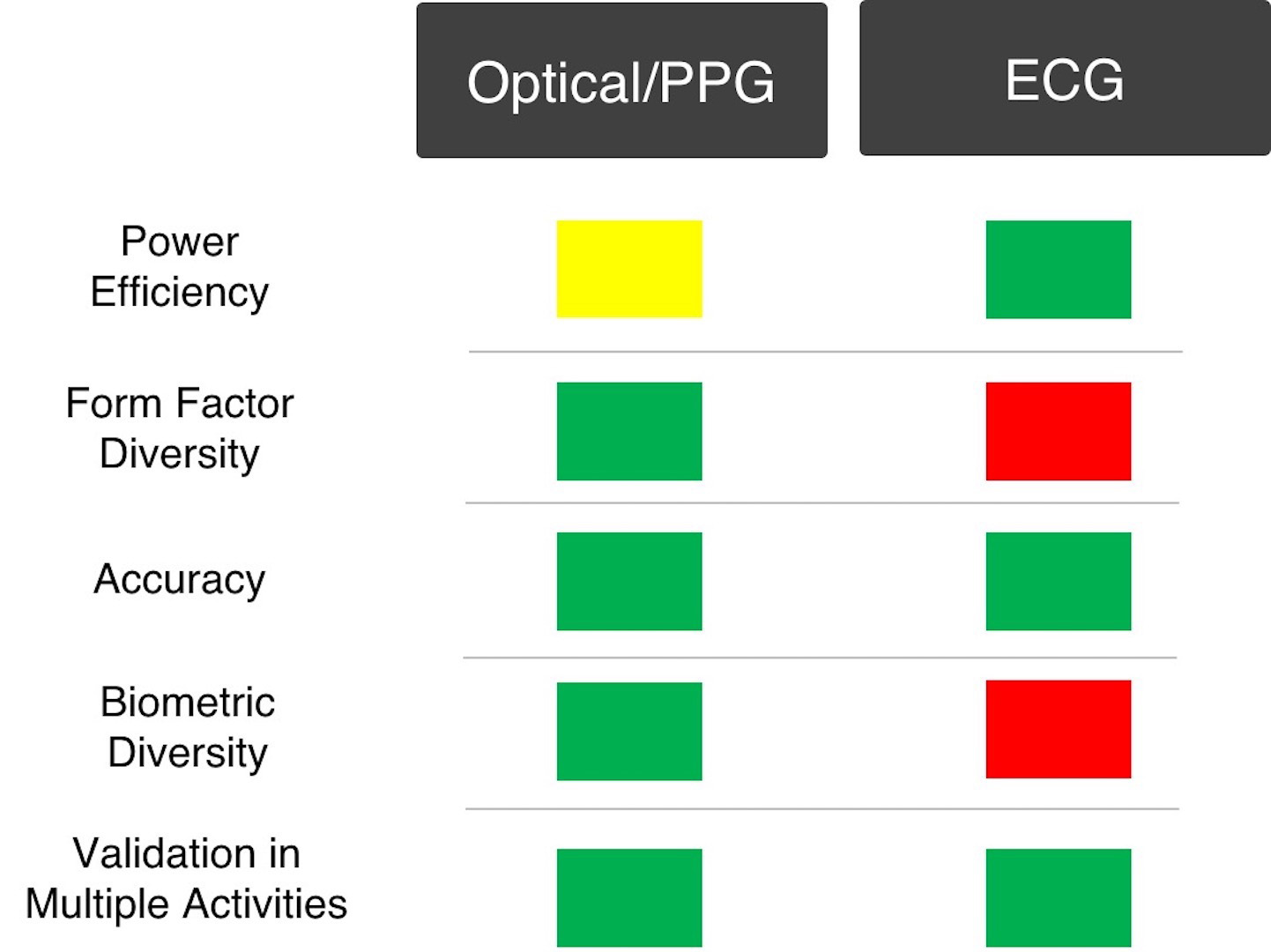
Table 1 – A Comparison of PPG and ECG sensors. (Image Credit: Dr. Steven LeBoeuf)
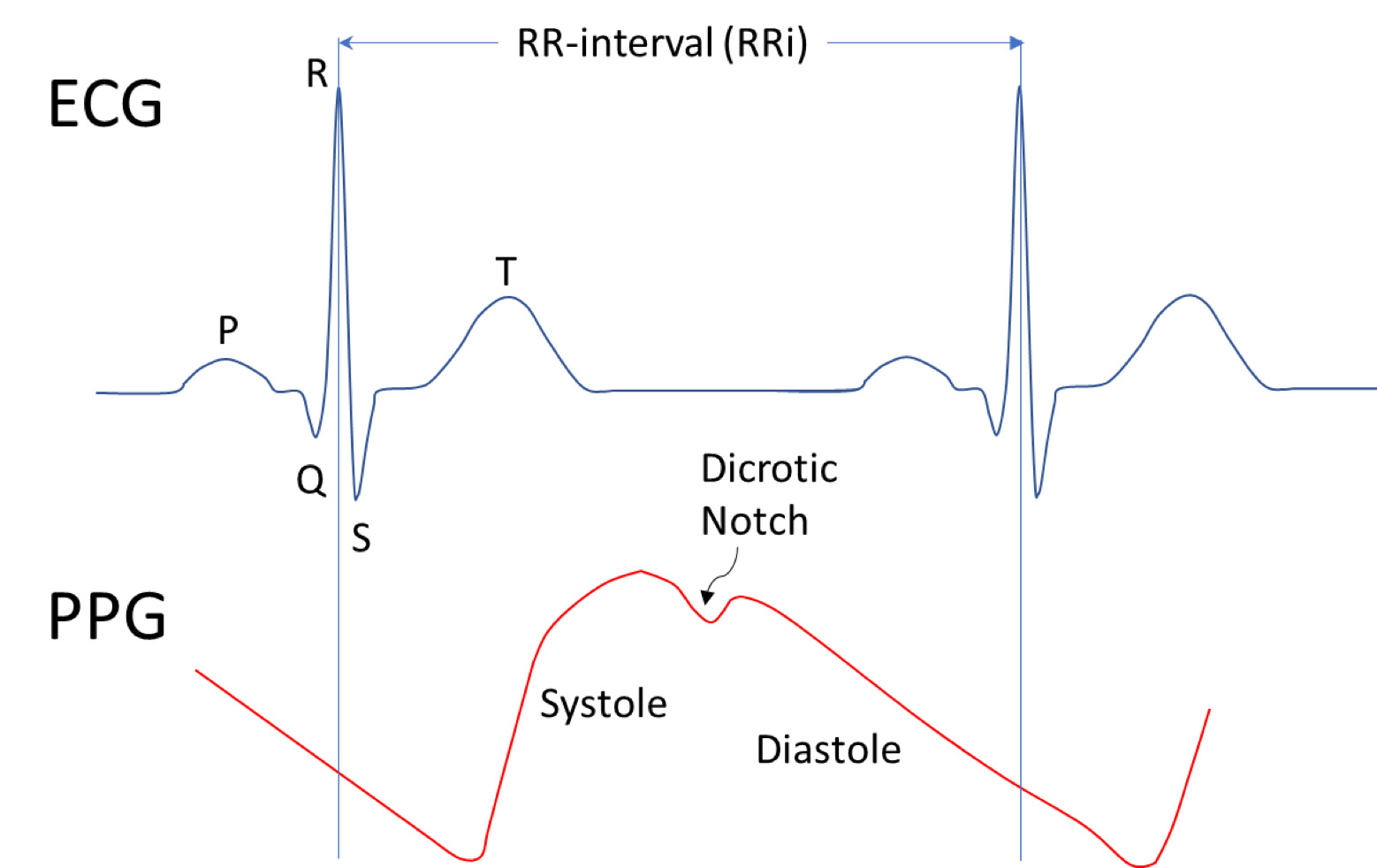
Figure 1 – PPG and ECG waveforms. (Image Credit: Dr. Stephen LeBoeuf)
“Form-factor diversity” is irrefutability in favor of PPG over ECG. PPG sensors can (in principle) work well on virtually any body location where there is high enough blood perfusion (where there’s skin.) For this reason, PPG sensors can be integrated into wristbands, earbuds, hearing aids, armbands, patches, rings, eyewear, and the like. In contrast, wearable ECG sensors are fundamentally limited to skin regions neighboring the heart, mostly because the electrical signal intensity drops off at a rate of 1/r2 with distance away from the heart. In order to get around this limitation, researchers have developed portable electrodes that can be placed across fingers on different hands, such that a substantial voltage drop across the right and left side of the body can be measured. This approach has been shown to work quite well using dry electrodes in smartphones. While this approach is not truly wearable, it is indeed highly portable and mobile.
PPG sensors win in terms of “biometric diversity” because they can support several types of biometric measurements. While both PPG and ECG have been shown to accurately measure heart rate and RR-interval (the time between successive heartbeats, as shown in the figure below, only PPG can be used to assess SpO2 (optically derived blood oxygen saturation), hemoglobin concentrations, blood constituent levels (such as bilirubin levels), and blood pressure. However, it should be noted that only ECG is capable of monitoring important electrocardiographic characteristics such as the P, Q, R, S, and T waves which can be extremely useful, or even critical, to accurately assessing medical conditions. For example, the detection of a short PR interval (the time between P and R peaks) is essential to properly diagnosing Wolff-Parkinson-White (WPW) syndrome.
One particularly promising growth area for both ECG and PPG-based medical wearables is cardiovascular screening, where biomarkers for disease conditions are detected to facilitate a medical diagnosis by trained professionals. Biomarkers that are particularly well-suited for medical wearables include blood pressure, cardiac efficiency, and arrhythmia — such as atrial fibrillation. These biomarkers can help assess the near-term risk for a heart attack, stroke, or cardiovascular failure, enabling preventive therapy to avert costly and life-threatening outcomes.
In particular, the recent consumerization of atrial fibrillation monitoring is a great example of how consumer biometric sensor technology has been successfully applied towards regulated consumer (over-the-counter) medical devices. Alivecor, a San Francisco mobile health startup, has been a trailblazer in the field of over-the-counter atrial fibrillation monitoring, with the FDA approval and launch of their Kardia mobile device.
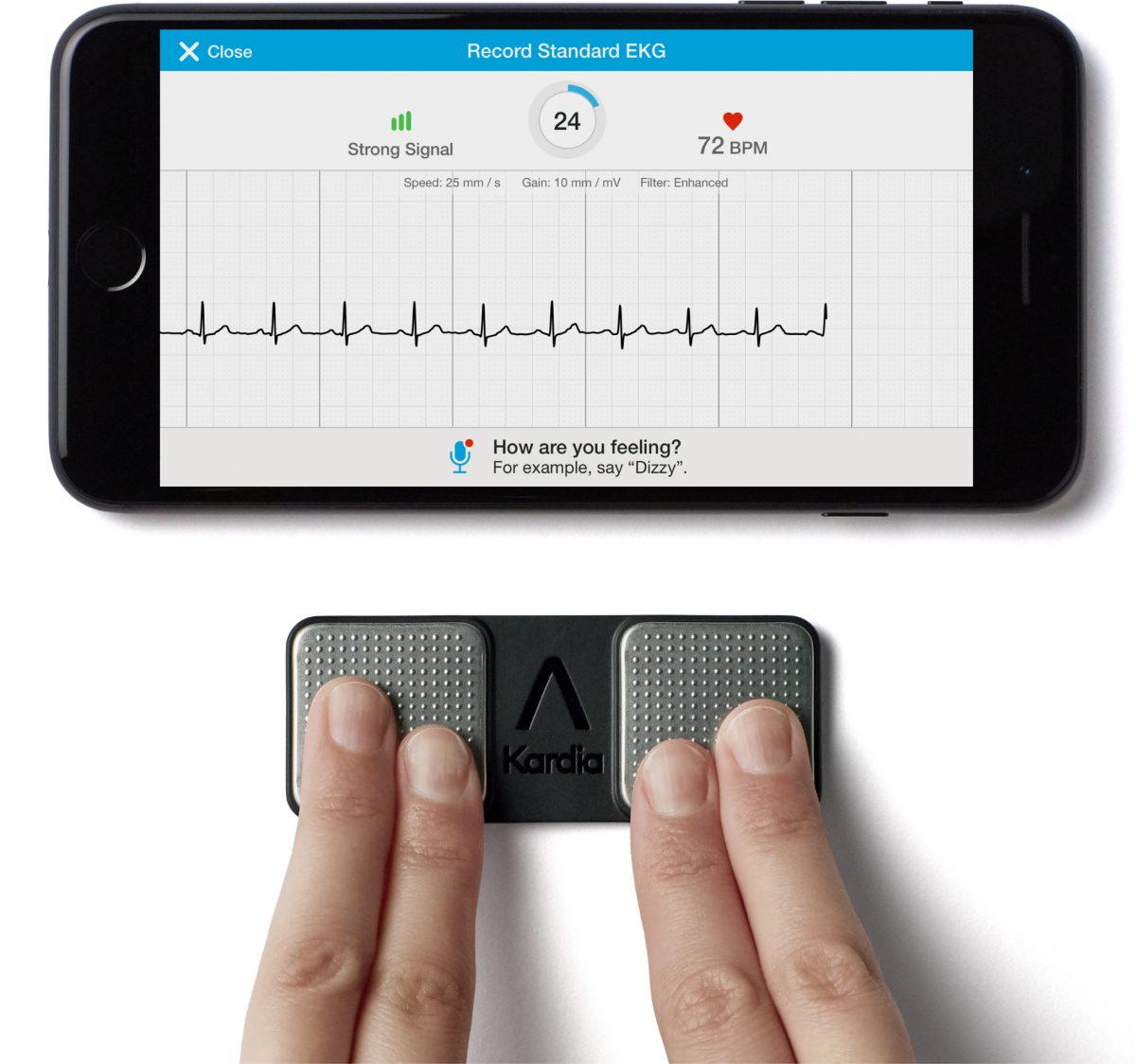
Figure 2 – The Alivecor Kardia mobile device. (Image Credit: Alivecor)
The Kardia device acquires ECG signals using a pair of dry finger electrodes (as described earlier), one for each hand. It passes its ECG measurements through a wireless link to a smartphone where an app processes them to detect atrial fibrillation. Similarly, researchers have validated accurate atrial fibrillation detection with PPG sensors located at various body locations.
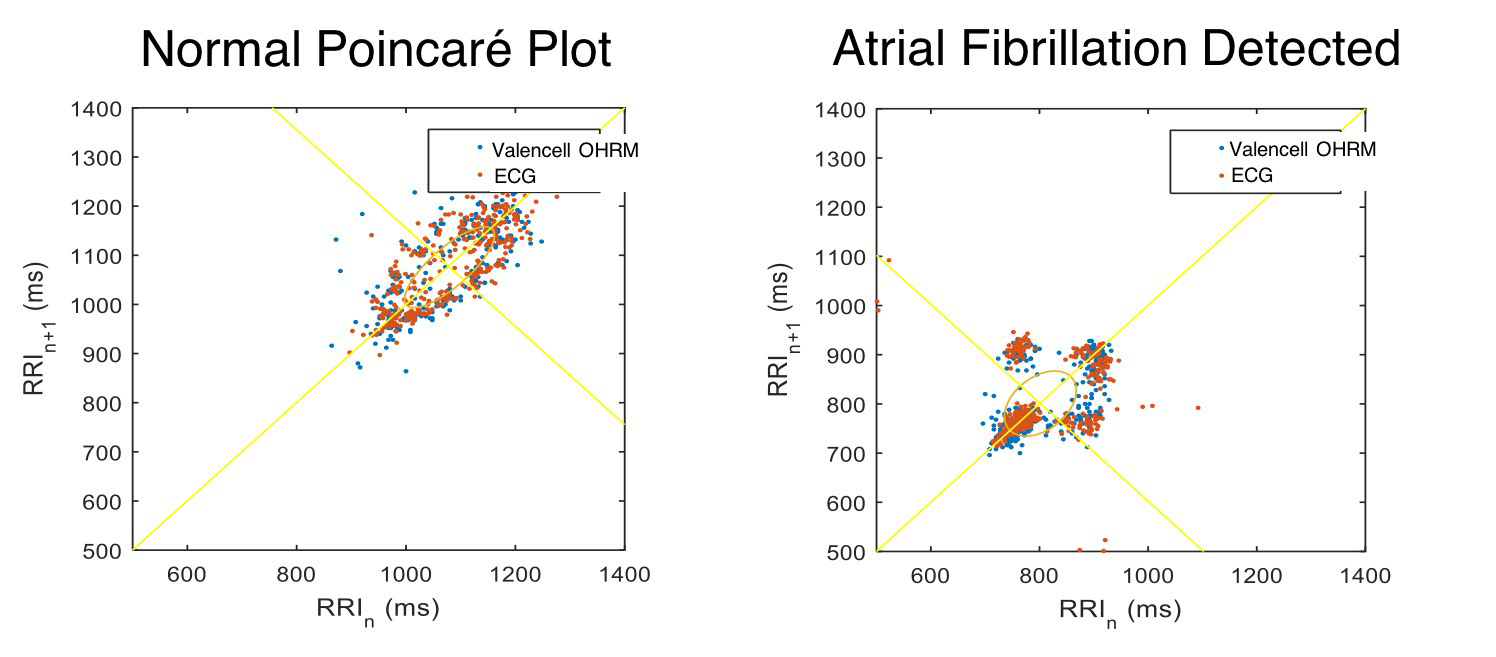
Figure 3- Normal and abnormal RRi plots of cardiac activity. (Image Credit: Valencell)
Although ECG and PPG are based on very different technologies (electrical vs. optical), the method of determining irregular heart rhythms can be nearly identical. For example, once an accurate stream of RR-intervals (see Figure 1) has been collected, either by method, they can be processed to identify statistical distributions associated with atrial fibrillation. One commonly used approach is the Poincare method of plotting each RR-interval versus the RR-interval that came before it. In fact, this approach has been shown to successfully detect atrial fi brillation in subjects with paroxysmal atrial fibrillation with greater accuracy than traditional 24/7 ECG monitoring. These findings agree with Valencell’s internal studies, where the characteristic “clustering pattern” has been observed in subjects having atrial fibrillation, as shown in Figure 3 below. This figure was created using the Poincare method, plotting RRi data from both an ear-worn PPG sensor (blue dots) and a Polar Electro ECG chest strap (red dots).
It should be noted that the Poincare method requires accurate RRi data to feed the model. Because the PPG waveform is not as “sharp” as an ECG waveform, it was once believed that accurate RRi data could not be generated by PPG sensors. However, this problem has been solved for wearable devices, and now Valencell provides commercially available PPG-based Benchmark Sensor Systems capable of accurate RRi monitoring. This significance is profound, as accurate RRi-monitoring via PPG enables wearable sensors located at virtually any location of the body. However, it should be noted that continuous accurate monitoring of RRi requires locating the wearable sensor at a relatively immobile body location. So for the foreseeable future, chest-worn patches and ear-worn-devices are likely to be the only body locations where continuous accurate monitoring can be achieved during daily life activities. While wrist-worn sensors can enable accurate spot-checking of RRi when a user is instructed to remain completely at rest for an acute measurement, the wrist is simply not a viable location for accurate continuous RRi monitoring in the free-living use case.
Since the conception of connected wearables for mobile health, the dream has been to make healthy lifestyles easier, more effective, and more affordable through seamless wearable technology. Advances in sensor technology are helping realize this vision with performance that enables the evolution from “mood ring” activity trackers to medically relevant solutions.




