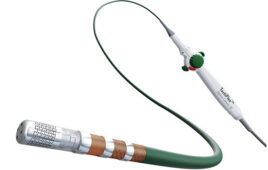
Reality study
Reality was designed to assess the safety and effectiveness of a vessel preparation strategy with directional atherectomy (DA) prior to DCB angioplasty for subjects with symptomatic severely calcified femoropopliteal peripheral artery disease (PAD).
Subjects across 13 multinational centers with 8-36 cm femoropopliteal stenoses or occlusions with bilateral vessel wall calcification were enrolled and treated with DA prior to DCB angioplasty. The primary effectiveness endpoint was 12-month primary patency and the primary safety endpoint was freedom from major adverse events through 30 days.
Among 102 subjects, 12-month primary patency rate came in at 77% and freedom from CD-TLR rate was 93%, with zero device-related or procedure-related deaths reported, although one index-limb major amputation was reported.