Deep brain stimulation is a long-established surgical procedure that eases the effects of Parkinson’s disease and essential tremor through pulses from tiny electrodes implanted in the brain. Currently, most surgeons around the world conduct this surgery while the patient is awake.
It’s considered standard clinical practice to keep a patient awake for the four to six hours it takes to implant electrodes into specific areas of the brain that control movement. Yet technological improvements in imaging have enabled neurosurgeons at OHSU to accurately map the brain before and during the procedure. This allows them to conduct the surgery while the patient is fast asleep.
“It doesn’t take a rocket scientist, or a brain surgeon, to tell you that patients prefer it,” says Kim Burchiel, MD, professor of neurosurgery in the OHSU School of Medicine.
The question is, do the clinical outcomes measure up?
New research indicates the answer is, yes. A clinical outcome trial of 69 people who underwent DBS surgery at OHSU, published in the journal Neurology, indicates that those who underwent the procedure while asleep experienced better clinical outcomes in terms of communication, cognition and speech. Further, there was no demonstrable difference in improvement of slowed movement, muscle rigidity or tremor between those who underwent asleep-DBS versus those who were awake during the procedure.
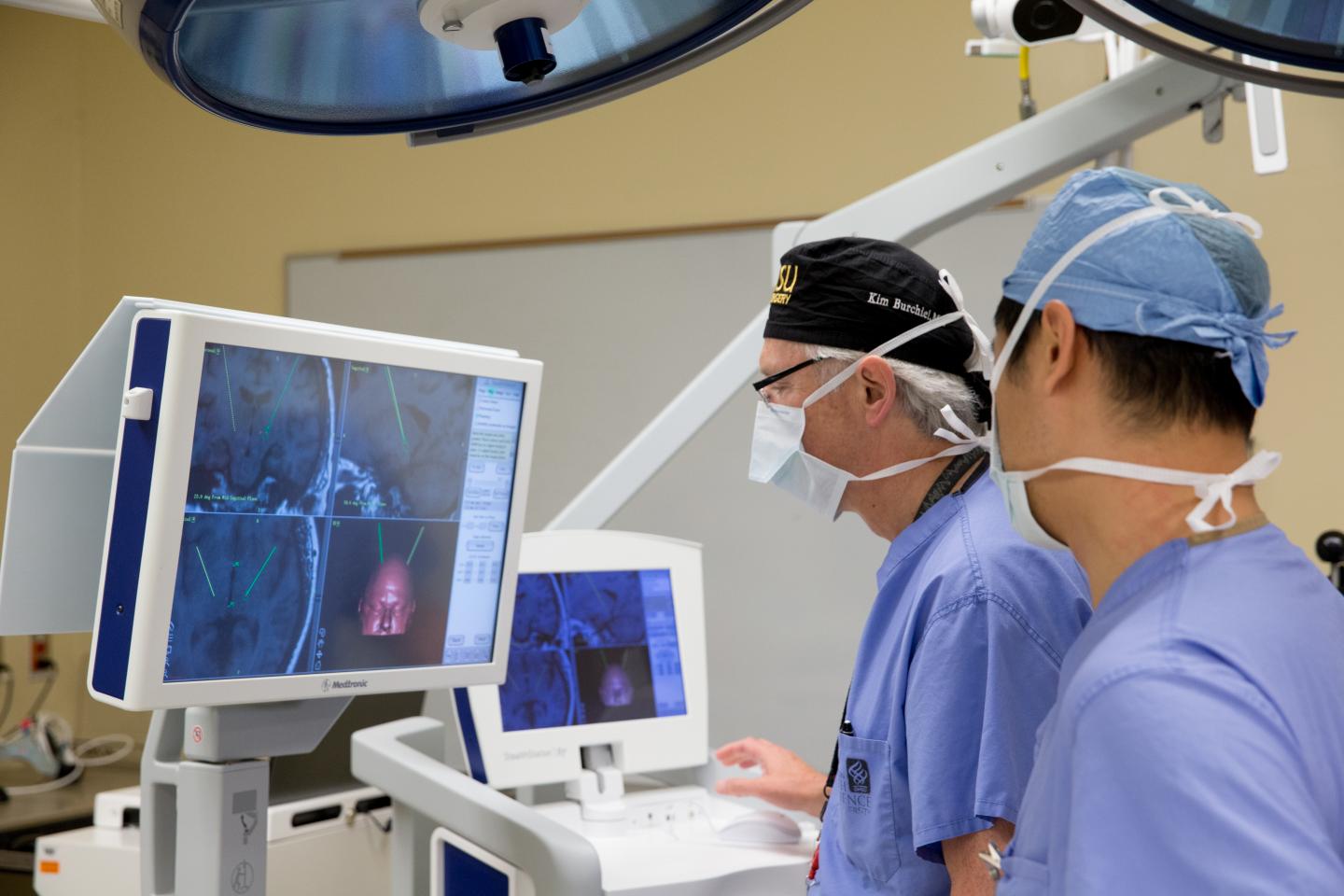
Kim Burchiel, MD, (center), past chair and professor of neurosurgery in the OHSU School of Medicine, conducts a deep brain stimulation surgery at OHSU. A new study reveals no demonstrable difference in clinical outcomes between surgeries conducted while the patient is asleep versus awake. (Image credit: OHSU)
Lead author Matthew Brodsky, MD, an associate professor of neurology in the OHSU School of Medicine and medical director of OHSU’s deep brain stimulation program, was specifically interested in improvements related to speech.
Although deep brain stimulation can dramatically improve motor function beyond what medications can offer, loss of speech fluency is a common side effect. Brodsky hypothesized that the cause may be due to the fact that awake-DBS requires surgeons to place multiple microelectrodes into brain tissue to identify the location and borders of the targeted region. Patients were tested for speech fluency following their procedures.
“Patients undergoing asleep-DBS had improved speech versus patients undergoing awake DBS, whose speech fluency predictably worsened,” Brodsky says.
Burchiel, a co-author on the paper, noted that OHSU stopped doing awake surgeries as of January 2011. The study compares patients who went through awake-DBS in 2010 with those who went through the asleep-DBS more recently. Burchiel performed the surgery in each case.
“If the patient’s outcome is not better or at least the same as awake-DBS, then you shouldn’t be doing it,” Burchiel says. “This article is really a watershed because we’re in a position to say, why would you ever do an awake surgery if you can get the same results or better when the patients are asleep – and the patients’ satisfaction is dramatically better?”
Although OHSU is in the minority right now, Burchiel believes there will be a stampede to asleep-DBS among neurosurgeons worldwide within the next few years. Burchiel carved out a reputation more than 25 years ago as the pioneer of deep-brain stimulation, as the first physician to conduct the surgery in the United States. The surgery is now conducted in roughly 100 medical centers nationwide, including OHSU.
The waking version of the procedure involves the use of microelectrode recording, or MER, to identify the target for placing the stimulating electrode within the brain. This occurs while the patient is awake, to answer questions and validate effectiveness. However, these microelectrodes effectively require the neurosurgeon to send probes into the brain at least twice – once to map the territory with the MER and once to place the battery-powered electrode that will be left in the brain.
“With asleep-DBS, there’s really just a single pass into the brain,” Brodsky says. “We haven’t had a single patient ask to have this done while they’re awake. To the contrary, we’ve had people come from all over the country to undergo asleep-DBS.”
Caitlin Evans was one of those patients.
The 56-year-old Seattle resident underwent deep-brain stimulation surgery in September, and she has no recollection of the procedure after anesthetization in the operating room. Twenty days later, after clinical staff programmed her electrode, she experienced immediate relief from the dystonia that had hobbled her since her Parkinson’s diagnosis in 2013. Once the electrode began working, she and her husband, Patrick Higgins, set out for a stroll across a Willamette River bridge near OHSU’s south waterfront campus.
“I walked comfortably for the first time in five years,” she says.
Evans realizes the procedure doesn’t cure Parkinson’s but only treats symptoms of her disease. Over the long term, she has reason for confidence that that OHSU’s scientists and physicians will further improve outcomes.
“Hopefully this will last a few years and I’ll get to enjoy myself,” she says. “Meanwhile, these guys will figure out how to fix me.”
In the meantime, the latest evidence points toward the benefits of DBS while patients sleep.
“Eliminating MER will reduce risks without compromising outcomes, shorten the procedure duration, and reduce its costs,” write Tipu Z. Aziz, FMedSci, and Marwan Hariz, MD, PhD, in an editorial in Neurology that accompanied the study. “Asleep surgery is certainly better for patients.”
The research in this study was funded in part by Medtronic.




