The O2Vent, designed for the treatment of snoring and sleep apnea, is being unveiled in Denver this month by Australian medical device company Oventus Medical.
It’s a 3D-printed titanium mandibular (jaw) advancement device custom-fitted to a patient’s mouth. Engineered with a separate airway, it directs air to the back of the mouth — bypassing obstructions in the nose, back of the mouth and tongue.The 510(k) cleared device, which was developed by Australian dentist Dr. Chris Hart, Oventus’ founder and clinical director, is designed to reduce or eliminate snoring and obstructive sleep apnea.
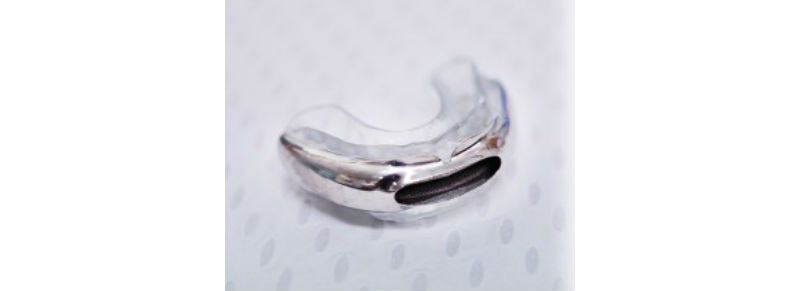
The O2Vent being unveiled this month. (Image courtesy of Oventus Medical)
A recent clinical study of the O2Vent in Australia demonstrated that snoring was eliminated in 82 percent of patients and significantly reduced in all 100 percent of patients in the trial. Positive results were seen in people who had nasal obstructions and mainly breathed through their mouths, including when they were asleep.
“The recent clinical trial data strongly showed that the O2Vent significantly reduced snoring and sleep apnea in most patients studied, even in those who historically did not benefit from other treatments due to chronic nasal obstruction,” Hart reported. “The trial also demonstrated that the device improves oxygen levels in most patients.”
He added that it also provides an alternative for patients who suffer from mild to moderate sleep apnea but who are CPAP intolerant.
In March, the device received FDA 510(k) clearance and the company expects it to be available for sale in the U.S. later this year. The O2Vent has been entered in the Australian Register of Therapeutic Goods (ARTG) since 2014 and is already on market there.
The U.S. Sleep Foundation estimates that 37 million Americans regularly suffer from snoring, while the U.S. National Institutes of Health says that 12-18 million American adults have sleep apnea, making the market here a significant one for anti-snoring devices, according to Oventus. The global sleep disordered breathing market is estimated to be worth $50 billion annually.
Oventus is preview it at the American Academy of Dental Sleep Medicine’s 25th Annual Meeting and at Sleep 2016, both of which take place in Denver.




