The issue and hazards of surgical smoke have long been discussed. So why isn’t everyone already on board?
As with many new concepts, these things take time. According to a review by The Journal of Hospital Infection, over 500,000 people are exposed to surgical plumes each year. These plumes can be the byproduct of electrosurgical or laser procedures and carry many potential hazards to surgeons, operating room staff and patients.
Have you ever wondered why your hospital mandates the removal of laser plume but not the smoke created by electrosurgery? Is electrosurgical smoke less hazardous?
Research by Dr. Yoshihiro Tomita and his colleagues note that when electrosurgery is used to vaporize one gram of tissue, inhaling the resulting plume would be like smoking six unfiltered cigarettes, while using a laser would be like smoking three unfiltered cigarettes.
So, why then are we still breathing in all of the toxins found in electrosurgical smoke when laser plume continues to be diligently evacuated?
Advocate groups like the Association of periOperative Registered Nurses (AORN) and the International Council on Surgical Plume (ISCP) are making headway, but without the incentive of government mandates, facilities make the decision for themselves.
This is why the presence of devices which remove surgical smoke from the air has become increasingly important as electrosurgical procedures have become more common.
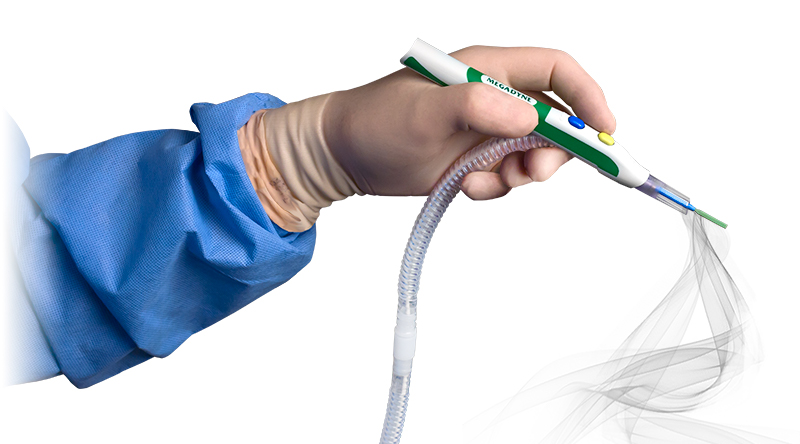
The ZIP Pen, manufactured by Megadyne, is an evacuation device that effortlessly and ergonomically removes surgical smoke for a healthier OR.
When it comes to limiting surgical smoke exposure, the risks are very real and the lack of action we’re taking is quite disconcerting. However, there are some specific things you can do to make your OR 100 percent smoke free.
How to create a smoke free surgical environment from the bottom up
So how does a surgical center or hospital achieve a smoke free surgical environment?
The most obvious place to begin is the point of service (those taking direct care of the patients, i.e., nurses, surgical techs, etc.), so the focus for initiation must begin with staff members.
Staff members are especially critical to the system’s ability to adapt and thrive. Enlisting their help in convincing colleagues that smoke evacuation is vital to their own health, along with patient safety, can be much more effective than a top down mandate.
Some strategies to create a smoke-free environment from the bottom up include the following:
1. Determine who the smoke-free champion will be and then give him or her responsibility and authority to initiate the smoke-free program.
2. Change physician procedure cards to reflect smoke evacuation supplies that are needed during specific procedures.
3. Put smoke evacuation supplies in custom packs.
4. Gain total support from staff members through education while stressing the negative consequences of smoke exposure. Providing testimonials from staff members who have developed respiratory problems from years of smoke inhalation in surgery can have a very powerful effect on others.
5. Convince surgeons to use smoke evacuation by providing the research to justify the use of smoke evacuation practices. Also recognizing a physician for being compliant with smoke evacuation guidelines can be very gratifying to some surgeons.
6. Help make the use of smoke evacuation devices and supplies an easy and seamless practice. Allow sales representatives to educate and train staff members until surgeons and staff members feel confident in their abilities and skill levels.
7. Encourage everyone in the OR to participate in the evaluation of smoke products. Document their responses, preferences, and rationale for acquiring specific devices and tally the results to determine the most popular devices. Standardization should be a common theme when purchasing smoke evacuation devices. This helps to ensure familiarization of products which, in turn, encourages compliance.
8. Recognize the success of implementing a smoke evacuation program in a hospital or department newsletter.
9. Gain leadership support through education, testimonials, and peer pressure to realize the far-reaching positive effects of a smoke-free OR.
10. Post signs to reflect hazards of surgical smoke to remind staff and physicians about the negative consequences of smoke inhalation.
11. Incorporate competencies for smoke evacuation whenever “skills days” are planned.
12. Make the implementation of smoke evacuation fun. For example, challenge the staff members by having a contest to encourage the use of smoke evacuation.
13. Actively address the identified barriers preventing smoke evacuation.
14. Select a “start date” to become totally smoke free and advertise this date to all staff members and physicians. Everyone can then work towards that goal.
How to create a smoke free surgical environment from the top down
If the decision is made to initiate the smoke-free OR policies from the top down, strong leadership is required. Leaders are designated as the agents of change and must remain knowledgeable and current on the federal, state, and local regulations that can have an impact on surgical smoke evacuation compliance.
Leaders must also make current and new research articles on the dangers of surgical smoke available, along with encouraging creative ideas on how to implement a surgical smoke evacuation program.
Being available to teach staff members about surgical smoke hazards and proper evacuation, or inviting experts to speak on the hazards of surgical smoke and compliance practices are also useful. In providing proper education and other opportunities, leaders are creating a sense of urgency among the staff members and physicians so that everyone will feel the need to evacuate plume.
The following are some more strategic plans to implement smoke evacuation policies from the top down in the OR suite:
1. Select champions who are adamantly involved with making the change to smoke evacuation so that they can mentor others.
2. Plan meetings for all of the stakeholders so that the message and the actions are consistent.
3. Create a budget that will provide financial support for supplies and devices to evacuate all surgical smoke.
4. Evaluate the outcomes of all efforts to achieve a 100 percent smoke-free environment in the OR. Make changes in strategies for implementation, as necessary.
5. Document comments and outcomes of the product evaluation of smoke evacuation devices and supplies. Use these data to make purchasing decisions.
6. Contact facilities that are 100 percent smoke-free to determine the reasons for their successes. Solicit creative ideas from staff members, physicians, and other stakeholders to become a smoke-free surgical arena. Implement those steps in creating a smoke-free program.
7. Send a letter to all operating physicians about the changes that would be made regarding surgical smoke evacuation. This will allow them time to respond if they have questions about the smoke-free program.
8. Tactfully handle those who don’t feel the need or urgency in evacuating smoke. Determine the reasoning behind their negative attitudes and non-compliance.
9. Provide a cost analysis of smoke evacuation devices and supplies as compared to worker and patient safety.
10. Treat the need to create a smoke-free surgical environment as a workplace safety issue. In other words, get risk management involved and stress that the OSHA General Duty Clause must be followed (the employer must provide a safe workplace environment for all employees).
11. Directly address barriers to noncompliance. Research has shown that more common barriers to noncompliance are: smoke evacuation devices and supplies are not available, physicians are resistant to change, the smoke evacuator is too noisy, or the staff is complacent and doesn’t feel the need to comply with existing smoke evacuation policies.
12. Partner with smoke evacuation vendors to help educate the stakeholders about the smoke evacuation devices and equipment and the ease of use.
13. Recognize the success of a smoke evacuation program with a letter to the department from the hospital or system president or CEO.
14. Create a written and verbal business case about the need to evacuate all surgical smoke and present it to the facility executives.
Whether plans are to implement the smoke evacuation program from the bottom up or top down, common strategies must be implemented for success to occur. A date should be set that can serve as a goal for all as to when the 100 percent smoke-free environment will be achieved.
Partnering with industry to be successful in becoming smoke-free is critical as industry partners can provide valuable education and training needed to convince staff members and physicians of the urgency to evacuate all smoke. This also helps to promote support from different groups as buy-in is extremely important for success.
Finally, a recognition and rewards program needs to be created to recognize those who have been instrumental in reaching the goal of achieving a smoke-free environment.
The good news is there are products from a number of device providers that remove surgical smoke from the air. This has become increasingly important as electrosurgical procedures have become more common.
When limiting surgical smoke exposure, the risks are very real and the lack of action we’re taking is quite disconcerting. However, if you take action and begin the process now, you can be well on your way to making your OR 100 percent smoke free.




