Spinal Simplicity, a medical device company developing innovative solutions to treat complex surgical problems, has received its third 510(k) clearance from FDA for the Minuteman G3-R spinal implant, part of the Minuteman family of supplemental fusion and fixation devices.
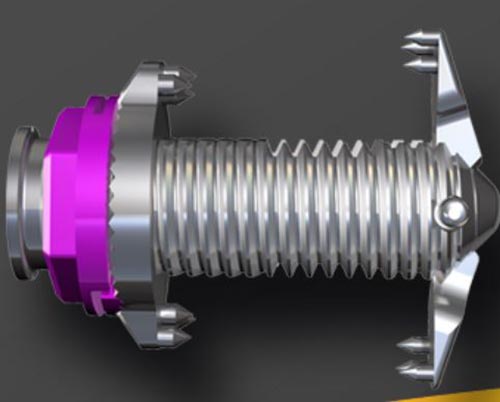
The Minuteman G3-R may be implanted via a minimally invasive lateral approach and will have a lower profile upon placement of the device.
(Credit: Spinal Simplicity)
“The lower profile of the Minuteman G3-R provides an aesthetic enhancement while maintaining the key elements of strength of the device and its minimally invasive nature. The interest that surgeons have expressed in this new product has been outstanding,” says Todd Moseley, Co-Founder and CEO of Spinal Simplicity. “Spinal Simplicity continues to raise the bar in the industry as we bolster our product portfolio and provide physicians with diverse options for patient treatments that utilize minimally invasive technologies”.
The Minuteman® G3-R is a sterile packed, posterior, non-pedicle supplemental fusion and fixation device for use in the non-cervical spine (T1-S1).
As an alternative to traditional pedicle screws, it is a plating system intended for supplemental fusion in patients with degenerative disc disease, spondylolisthesis, trauma and tumor.
The device is available in the U.S. with hydroxyapatite coating and a removable 12mm portion of the implant body.
The Minuteman is intended for use with bone graft material and is not intended for stand-alone use.




