Stryker announced that its AVAflex Balloon System has received FDA 510(k) clearance and is, for the first time, available with Stryker’s market-leading bone cements and implants and the AutoPlex Mixing and Delivery System.
“Doctors who perform vertebral augmentations are committed to the health and wellness of their patients, and Stryker is committed to empowering those doctors to provide the best possible care,” says Chad Ludwig, marketing director at Stryker Instruments. “The AVAflex Balloon System enables doctors to achieve bipedicular results with a unipedicular approach to vertebral augmentation.”
The AVAflex and AutoPlex systems are used in the treatment of vertebral compression fractures (VCF), which affect an estimated 750,000 Americans each year. VCF patients can suffer from extreme pain and are at an increased risk for serious health problems. Vertebral augmentation, including the use of a balloon system, has been shown to provide patients with significant pain relief and dramatically reduce mortality rates.
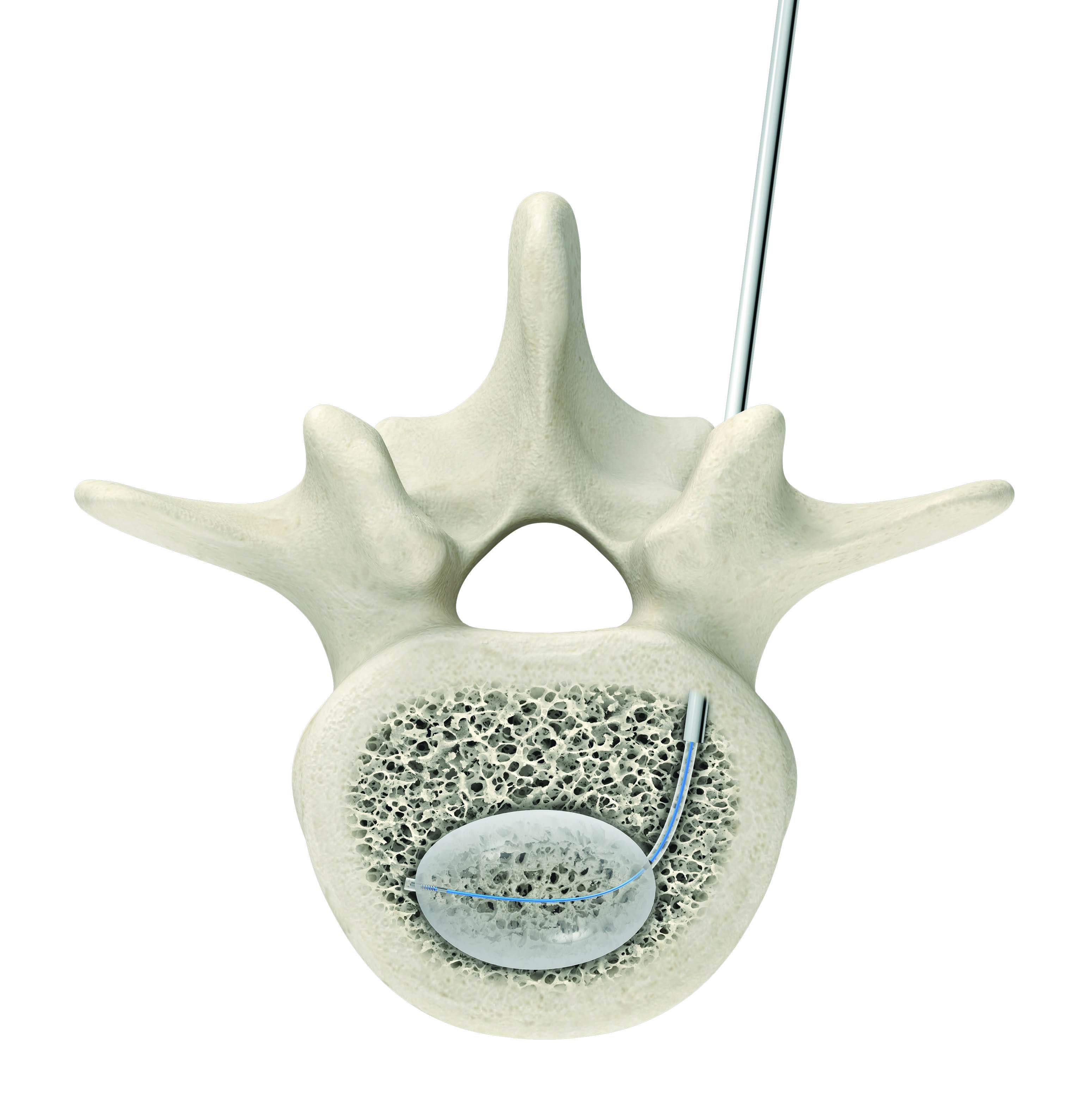
(Image credit: Stryker)
The AVAflex curved balloon system’s new 11-gauge size allows surgeons to achieve with one insertion and a smaller needle what had previously required two insertions, making procedures less invasive and potentially reducing the risk of patient trauma. Using a minimally invasive technique, physicians can successfully create a midline cavity for targeted cement placement by accessing one pedicle.
AVAflex is now available with Stryker’s bone cements and implants and the AutoPlex Mixer and Delivery System, an easy-to-use bone cement mixing and delivery system. The AutoPlex system provides consistent and thorough blending of components, helping eliminate human error and variability.
Stryker has furthered its mission of making health care better for physicians, hospital staff and patients with the addition of the AVAflex portfolio, which it acquired from Becton Dickinson in 2016. Stryker provides the most complete and least invasive portfolio of vertebral compression fracture treatment options.
With an unrivaled collection of balloon catheters and augmentation options, cements, automated mixers and directional delivery systems, Stryker enables care providers to tailor their approach for the treatment of vertebral compression fractures. Stryker is now the exclusive provider of automatic mixing and delivery systems and 11-gauge curved balloons.




