
Jim Jarrett | Founder & President | AIM Plastics’ Tech Center
Need more than a prototype, but not ready for a high-volume production mold? Take a look in the middle. Hybrid or bridge tooling may be your answer.
Over the past decade, there have been significant advances that greatly affect traditional prototype and low-volume mold construction. It’s not a one-key-fits-all solution anymore, so here’s some clarification and direction to help navigate through the mold maze. First things first: Address the basics.
1. What is the estimated yearly volume?
2. What is the lifespan of the program?
3. What resin will be used?
With this information, along with a 3D CAD file and printout identifying all critical dimensions, a reputable mold builder or molder can quote a best-fit tooling solution that will achieve quality requirements and provide a tool life that doesn’t fall short of the program lifespan. Companies don’t want to pay for a 1-million-part tool life and incur a 12-week lead time when only 150,000-tool life is required with a much faster lead time.
A prototype or low-volume molder should have all the protocols and procedures of a production molder, so audit and select one carefully. The molder should offer in-house mold construction and have experience with hybrid and bridge tooling. In most cases, this can fill prototype requirements, allowing engineering changes to move on through validation and meet production needs.
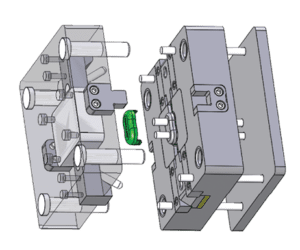
A Solidworks image of a single-cavity hybrid showing the options and benefits of this type of tooling.
Mold construction
Having the tooling built in-house (where the mold builder and molder are the same entity) is preferable, as it eliminates finger-pointing and helps keep to tight timelines.
Mold design software has come a long way, with draft analysis and parting line split tools allowing quick, efficient design for manufacture. Additionally, tools such as quick split and electrode creation save the designer time, enabling a quick and efficient 3D mold to be placed on the shop floor for construction within days.
Shops with high-speed machining centers have two major benefits:
(1) They can cut hard steel, eliminating many post-heat-treat procedures while maintaining a superior surface finish, which reduces benching and polishing time.
(2) With high spindle RPMs, small cutter diameters can be used in many cases, omitting the need for Electrical Discharge Machining (EDM) procedures, both decreasing deliveries and cost. Current CNC EDM machines offer benefits for quick-turn mold construction as well. Their metal removal rate is much faster than older manual machines and leaves a better surface finish, reducing or eliminating the need for benching or polishing procedures. In most cases, light texturing such as MT11020 is achieved with an equivalent EDM finish, eliminating the need for costly texturing procedures and reducing deliveries as well.
Molding
The molding equipment should have gone through an install qualification/operational qualification process, and some type of standard validation procedure should be in place. Scientific molding practices should also be followed. All basic production procedures should be in place as well as all employees following the cGMP guidelines.




