Contract manufacturer Switchback Medical (Maple Grove, Minn.) today announced that it has added a new division.
Contract manufacturer Switchback Medical (Maple Grove, Minn.) today announced that it has added a new division.
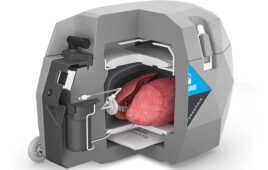
Switchback BioSim Innovations offers functional dynamic biosimulator model development, cell culture services and physician training, according to the company. Biosimulator models use animal and human tissue to create functional models, such as a pumping heart with working valves to assess a valve repair device, perfused tissues to evaluate neurovascular catheter usability, or a neuron cell culture built on a biodegradable scaffolding.
The new service allows Switchback to develop preclinical models for device testing and training that currently do not exist, improve the safety and efficacy of the devices and significantly speed the development cycle, according to the company.
“We are extremely excited about this new venture,” Switchback Medical principal said Brady Hatcher in a news release. “It allows our customer’s product development teams to get more clinically relevant and faster testing feedback. With the BioSim Innovations capability, we can now host development through physician training all here in one location.”
BioSim Innovations can create models that simulate pathological conditions that can be repaired by the devices being developed, according to Switchback. The increased sophistication of medical devices being invented requires improved models and training solutions that current benchtop or animal models do not achieve, the company added.
“We are excited to help our customers design experiments that generate data demonstrating the efficacy, usability and risk analysis associated with their device, which will also allow us to offer a better physician training experience for complex devices,” said Dr. Leanne Bakke, director of BioSim Innovations. “This new offering creates a sandbox approach for scientists, engineers and physicians to innovate together.”