Synopsys announced today that it launched a new software version to update the Simpleware ScanIP Medical program with FDA 510(k) clearance.
Synopsys announced today that it launched a new software version to update the Simpleware ScanIP Medical program with FDA 510(k) clearance.
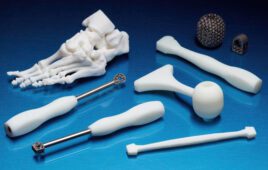
According to Synopsys, the new software includes 3D medical printing and point-of-care 3D printing, making Simpleware ScanIP Medical one of just a handful of software programs to garner such clearance with 3D-printed models created through the program suitable for diagnostic uses.
The software allows for processing medical imaging data into 3D models for pre-surgical planning and 3D printing, generating watertight STLs for direct export to 3D printing applications, according to the company’s website.
Synopsys’ platform offers patient-specific images through the combination of the image and CAD data for evaluating implant positions, while the tools on hand allow for the visualization and measurement of medical image data.
The company touts the platform as easy to learn and use with an intuitive interface, accurate and reliable through its high-quality anatomical models and customizable through automated repeatable tasks and operations with scripting and plug-ins. Additionally, the software is print-ready with a direct link to bundled 3D printers, fully supported with licensing from Synopsys’ expert team of application engineers and it received FDA, CE mark and ISO certifications.
In March, Synopsys announced the launch of the Simpleware R-2021.03 fully automated segmentation and landmarking tools. The AI-enabled software is made for new anatomies to improve orthopedic and cardiology-based workflows.
The modules are a one-click solution to help reduce tedious manual work on medical image data while producing models in 1-3 minutes with a standard engineering laptop. The software is run on local hardware to eliminate the need to upload confidential patient data to an offsite server for processing.