Kevin Parmenter, VP, Application Engineering, North America, Excelsys Technologies
A power supply cannot be “certified;” however, it can be designed and tested to meet the necessary levels of performance according to the applied parts’ intended applications. A case history here provides one company’s experience during such testing.
The international standard IEC 60601 specifies testing performance for electrical devices used in medical applications. This standard has undergone significant changes in the last number of years, with the recent adoption of the 3rd edition of the standard in 2012. In fact, IEC recently announced planned introduction of the 4th edition of the IEC60601 standard, slated for adoption in 2018, making it a design consideration for the next generation of medical equipment designs.
Because the power supply is considered a component of a medical device, it is an applied part–meaning the medical electronics end-product–that must be evaluated for overall performance to meet a standard’s requirements. Although a power supply cannot be “certified,” it can be designed and tested to meet the necessary levels of performance according to the applied parts’ intended applications. Testing power supplies to this medical standard can help manufacturers optimize their medical designs to achieve the highest performance and safety levels and thereby enable first-pass success during agency approvals.
This article also describes how Excelsys Technologies Xsolo power supplies have been designed and tested to meet the B and BF type requirements per the IEC 60601 standard.
Safety classifications for medical applied parts
In July 2014, the IEC updated the medical standard IEC 60601-1-2 as related to “Medical electrical equipment: Part 1-2: General requirements for basic safety and essential performance; Collateral Standard: Electromagnetic disturbances; Requirements and tests.”
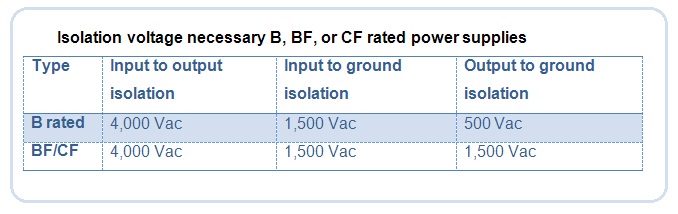
The 60601 standard cites three classifications for meeting these requirements: CF, BF and B. The requirements for leakage current for these classifications are in two tables that follow, which are taken from the IEC 60601 specifications.
The Cardiac Floating (CF) classification is the most demanding performance level. Applied parts falling under this rating have application in products including, but not limited to, ventricular assist devices (VADs) and dialysis machines. In these applications, electrical connection can be made directly to the patient’s heart during the operation of the device.
The Body Floating (BF) classification is less stringent than CF and intended for applied parts which have conductive long-term contact with a patient. These products can include incubators, patient heating and cooling equipment, ultrasound monitoring, cardiac monitoring and long term diagnostic equipment, and blood pressure monitoring, to name a few. The specification refers to Type BF and CF as floating because they are not connected to earth ground.

Test circuit 1 is from IEC 60601 and measured the circuit for patient leakage current, from patient connection to earth.
The least stringent requirement is for the type B or Body classification and is reserved for applied parts that are not conductive and can be immediately released from the patient. This includes, but is not limited to, LED lighting, medical lasers, hospital beds and medical imaging equipment such as MRI and phototherapy equipment. Type B applied parts may be connected to earth ground.
Testing power supplies to the medical standard
Our company developed its XS series of power supplies for medical applications. Testing the power supplies to the 60601 standard was undertaken using its basic and low-leakage models, the XS1000-48N-000 and XS1000-48N-004, respectively.
The high efficiency XS1000 Power Supply delivers up to 1,008W in an enclosed, fan-cooled chassis. Nominal output voltages are 24 and 48 V with wide adjustment ranges and user defined set-points. Xsolo carries dual safety certification, EN60950 2nd Edition for Industrial Applications and EN60601-1 2nd and 3rd
Edition for Medical Applications, meeting the stringent creepage and clearance requirements, 4,000 Vac isolation and less than 300 µA leakage current. With up to 92% efficiency the XS1000 is ideal for use in acoustic sensitive medical applications, harsh industrial environments, laboratory equipment and HI-Rel/MIL-COTS applications.
Excelsys used the isolation voltage ratings of each power supply to determine which one would be suitable for a particular application type. Power supplies vary widely in terms quality and reliability. There are design differences, depending on the supplier, and many power supplies are not meant for medical applications.
Test results

The circuit measures circuit patient leakage current which is caused by an external voltage on the patient connection of an F-type applied part.
Excelsys’ testing was performed in accordance with the IEC 60601 requirements using an AC source providing 264 Vac at 63 Hz, which demonstrates worse-case conditions. The power supplies were tested following by the IEC’s specifications, as shown in Test circuits 1 and 2.
The resulting data appears in Tables 4 and 5 along with the requirements in the specification showing the limits for AC testing. These results indicate that the XS – Xsolo products would be well suited to applications in either type BF or B applied applications.
Test 1: Patient connection to earth
For the first test, Excelsys used power supply settings of Vin = 264 Vrms (maximum rated input voltage) and a frequency of 63 Hz (maximum rated input frequency). The test method was to follow the instructions shown in Test circuit 1.
Test 2: Patient connection of an F-type applied part. Result: Pass
For the second test, Excelsys used power supply settings of Vin = 264 Vrms (maximum rated input voltage) an a frequency = 63 Hz (maximum rated input frequency). The test method was to follow the instructions shown in Test circuit 2.
Summary
Excelsys Technologies’ Xsolo power supplies are designed and tested to demonstrate that they are suitable for use in products intended for medical applications requiring compliance with either B or BF classification.
Moreover, it is not uncommon for applied products needing type CF performance levels to need additional isolation methods such as additional isolated-power converters in the system or the addition of medically rated, high-isolation transformers in the overall system design. The company makes available low leakage power supplies, which enable incorporation of multiple power supplies into various medical type rated systems depending on the application.
Excelsys Technologies
www.excelsys.com







![A photo of the Medtronic GI Genius ColonPro polyp detection system flagging a potential sign of colon cancer during a colonoscopy. [Photo courtesy of Medtronic]](https://www.medicaldesignandoutsourcing.com/wp-content/uploads/2024/04/Medtronic-GI-Genius-doctors-268x170.jpg)

