The controversy over the safety of blowing-air devices like 3M’s Bair Hugger and Stryker’s Mistral Air now involves the Centers for Disease Control and Prevention (CDC). With more than 800 victims of orthopedic implant infections suing Bair Hugger in multi-district litigation centered in Minnesota’s federal district court, a CDC advisory committee has recently weighed in.
The minutes of Healthcare Infection Control Practices Advisory Committee (HICPAC) November 2015 meeting, although focused on heater-cooling units used in cardiac surgery, offered advice more broadly, urging, “It is important not to blow air in the operating theater,” and adding, “Nothing that blows air should be in an operating theater, if possible.”
HICPAC is a CDC committee that makes recommendations regarding infection control.
In March 2016, the CDC reiterated the message — this time speaking specifically to healthcare facility purchasing departments. In a published recommendation, agency experts Michael Bell, MD, and M. Shannon Keckler, PhD, presented “Issues to consider for equipment used in high-risk locations, based on where they are used as well as who might be exposed,” specifically noting, “Air current generation: Devices that blow air should probably not be situated in high-risk locations.”
Although the CDC/HIPAC advice did not mention Bair Hugger or other forced-air warming (FAW) devices by name, the 3M device blows more than 40 cfm of air along with 950 watts of waste heat.
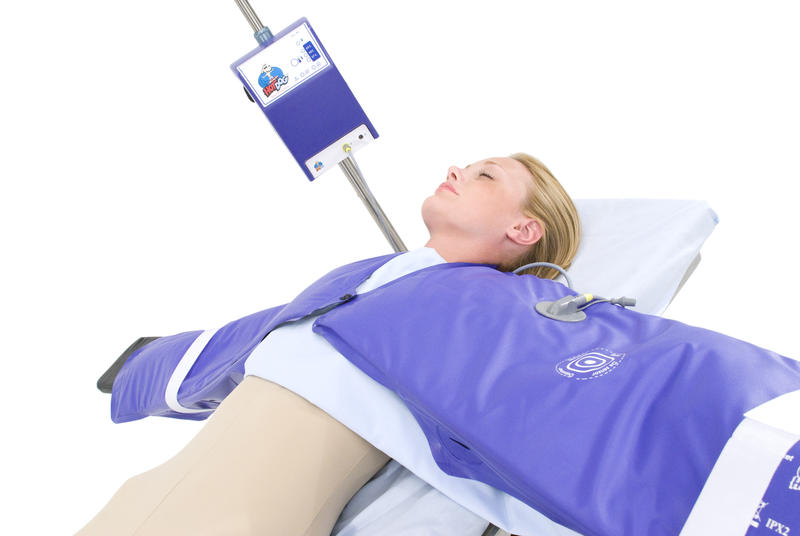
Products in the HotDog line offer an alternative to forced-air warming. (Image credit: HotDog)
The CDC-published Emerging Infectious Diseases Journal, while noting that research has not yet been done to definitely link FAW to specific infections, concluded, “Our evidence also highlights the risks associated with devices that generate airflows in the operating room and that were not part of the original operating room air management design.
“Until more detailed evidence is available regarding this issue…devices that generate drafts should be banned from the operating room.”
Critics of forced-air devices point to nine published studies corroborating the CDC’s conclusions and showing that the waste heat from FAW contaminates the sterile surgical field. For example,
peer-reviewed articles published in such journals as the American Journal of Infection Control, The Journal of Bone and Joint Surgery and Anesthesia & Analgesia have established that 2,000 times more contaminant particles were found in the air over the wound with FAW than with air-free conductive warming.
With forced-air warming, mean temperatures were significantly elevated over the surgical site, which made one study’s authors conclude, “Forced-air warming generates convection current activity in the vicinity of the surgical site.”
One of the studies, a retrospective review of 1,437 cases conducted in a U.K. orthopedic hospital, showed a 74 percent reduction in periprosthetic infections when FAW was discontinued in favor of air-free warming. A review article by Professor David Leaper, former Chair of the SSI Committee for The National Institute for Clinical Excellence (NICE) states, “We conclude that FAW does contaminate ultra-clean ventilation.”
Pathogenic contamination by convection currents is not FAW’s only problem. One study showed that micro-organisms were cultured from the internal air-flow paths of 100% of the forced-air blowers tested. Another showed that 100% of the blowers were emitting internally generated particles >0.3 μ m in size, up to 112,000 particles per ft3 of air — 300 million particles per hour.
Despite the published research, 3M strenuously and consistently argues that the Bair Hugger technology is safe. In a recent company release, specifically positioned as a “Response to Misstatements in Lawsuits,” 3M makes their position clear.
“There are many factors that are known to increase the risk of surgical site infections, including having other medical conditions such as diabetes, high blood pressure or heart disease, being elderly or overweight, and smoking,” the release says. “There is absolutely no evidence that Bair Hugger warming therapy causes or increases the risk of surgical site infections.”
There’s also no question that temperature management is vital during surgery. Without some sort of warming system, patients are highly susceptible to complications, including SSIs that can have fatal outcomes. 3M is quick to note that the Bair Hugger is the industry standard for this task and has been safely utilized in millions of procedures.
Scott Augustine, MD, founded Augustine Temperature Management, LLC in 2005 and along with his team invented and developed HotDog warming. ATM is a subsidiary of ABD, formed to commercialize the HotDog technology.
A version of this article appeared in the November/December 2016 issue of Surgical Products.




