Duodenoscope reprocessing
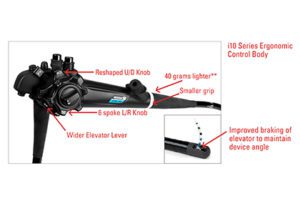
C.A.P. HD Duodenoscope [Image from Pentax]
The tools, though, are complex with many small working parts. Reprocessing is tricky. By 2015, there were alarming reports about deadly “superbug” infections potentially associated with the devices. FDA eventually warned the three major duodenoscope makers — Olympus (TYO:7733), Fujifilm Holdings (TSE:4901) and Hoya’s (TYO:7741) Pentax subsidiary — and ordered them to conduct post-market surveillance studies about the effectiveness of scope reprocessing.
Last year, FDA once again warned the three companies, saying they were failing to comply with the order. Dr. Jeffrey Shuren, director of the FDA’s Center for Devices & Radiological Health, this month said there would be possible “additional action” if deadlines are meant.
FDA, in fact, said this month that the problem is worse than previously thought, with a contamination rate for high-concern bugs like E. coli and Pseudomonas aeruginosa that’s around 5.4%.
Late last year, Olympus and a former senior executive in Japan pleaded guilty in federal court in Newark, N.J., to failing to file required adverse event reports involving infections connected to duodenoscopes. A previous LA Times and U.S. Senate committee investigation found that Olympus for years did not disclose problems to FDA and the U.S. public, even as it issues warnings in Europe.