Designed to detect speech and swallowing problems, the device has found a new use in tracking cough frequency to alert healthcare providers that frontline workers may need to be tested for SARS-COV-2.
(Image courtesy of Northwestern University)
Researchers in Chicago have adapted a flexible patch they developed to monitor stroke patients for swallowing trouble to help detect symptoms of COVID-19.
They’re hoping it can help physicians decide whether frontline healthcare workers have developed symptoms of the novel coronavirus so they can prevent the illness from worsening. In their “Lost on the Frontline” series, Kaiser Health News and The Guardian have reported 922 U.S. healthcare worker deaths that likely stemmed from caring for COVID-19 patients.
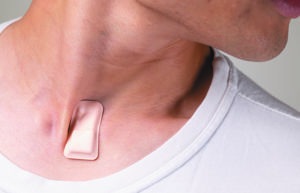
The device that might help identify the virus is a postage-stamp-size wearable that adheres to the skin at the base of the neck — an indentation known as the suprasternal notch — and generates data for up to 40 hours straight without needing to recharge.
Northwestern University biomedical engineer John Rogers had been working with Arun Jayaraman, a research scientist at Shirley Ryan AbilityLab, on developing the device to detect speech and swallowing problems in stroke patients and convey the data it collected to the patients’ physicians. Chicago-area healthcare providers from the who knew about their research asked if they could adapt the device to detect COVID symptoms.
“When COVID hit, we were overwhelmed by requests from the medical community here to adapt and customize that device to assess the key symptoms of COVID-19, which are fever, shortness of breath and cough,” Rogers said.
Rogers and Jayaraman figured that if the device could detect swallowing from placement on the neck, it could detect coughing. The COVID version of the device monitors coughing intensity and patterns, chest wall movements (which indicate labored or irregular breathing), respiratory sounds, heart rate and body temperature, including fever. It wirelessly transmits data to a HIPAA-protected cloud, where automated algorithms produce graphical summaries tailored to facilitate rapid, remote monitoring.
“We anticipate that the advanced algorithms we are developing will extract COVID-like signs and symptoms from the raw data insights and symptoms even before individuals may perceive them,” said Jayaraman, who is leading the algorithm development. “These sensors have the potential to unlock information that will protect frontline medical workers and patients alike — informing interventions in a timely manner to reduce the risk of transmission and increase the likelihood of better outcomes.”
Once the worker removes the patch at the end of a shift, the device sends the data to the physician to evaluate for COVID symptoms, according to Rogers. The device, which Rogers and colleagues have been manufacturing in a Northwestern lab, is in use in healthcare settings as well as in home settings for patients who’ve been discharged after a COVID-related hospitalization.
“The nighttime recordings are really important,” Rogers said. “A lot of people aren’t aware of how often they’re coughing during the night. Those recordings, along with other features of the data, have provided some very important health insights.”
For example, a nurse who caught the virus from her husband was admitted to Northwestern Memorial Hospital, where she agreed to wear the patch.
“We were able to supply her with a device shortly after she was admitted,” Rogers said. “She came very close to being transferred to the ICU and placed on a ventilator, but recovered to an extent that eventually allowed her to be released from the hospital.”
The nurse wore the patch at home for two more weeks after discharge from the hospital. There, it picked up very brief but extreme tachycardia events and apneas that were previously undiagnosed, Rogers said.
The researchers have $1.5 million in federal funding from BARDA for device development and continue to refine its capabilities. They have applied for FDA clearance rather than an emergency use authorization, which would only last for the duration of the public health emergency.
For now, they’re producing up to 100 devices per week, but they have a company in place, Sonica Health, that can take over that aspect of the work if] the FDA clears the device. The initial target markets are frontline workers, patients, military and elderly populations.
“There’s been a lot of interest,” Rogers said. “We’re trying to be responsive to requests rather than marketing the devices. We’re funded at levels that allow us to refine and develop and customize the technology at a high level, but our orientation is not around maximizing revenues and profits and related business aspects, but rather toward providing something of value to the broader community.”



