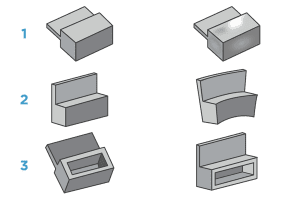
From left to right, Figure 1 represents a part designed with thick features and the resulting sink once molded. Figure 2 also shows a part designed with thick features, but this time the warp that occurs once molded. Image 3 demonstrates how coring out thick features helps create an optimally molded part.
Adapted from 13 Cosmetic Defects and How to Avoid Them by Kip Hanson
Innovation is speeding up, which means designers are experiencing a truncated design phase. As they are encouraged to design quickly, it has become critical to think about the elements of form and function, including cosmetics early in the planning.
As with any manufacturing process, injection molding comes with its own set of design guidelines, and design engineers who understand these best practices will increase their chances of developing structurally sound and cosmetically appealing parts and products.
Here are some common cosmetic defects that occur on plastic injection-molded parts, and tips on how to avoid them:
Sink
As its name implies, sink appears as a dimple or shallow depression on the surface of a molded part. It’s caused by thicker than normal cross sections, non-uniform part design or an improper gate placement. Some plastics are susceptible to sink, whereas fiber and glass-filled materials are less prone. Generally a workpiece minimum wall thickness be no less than 40% to 60% of its thickest section. Material flow within the mold should travel from thick to thin whenever possible, which might mean reorienting the mold cavity, or placing the gate in an area originally reserved for a cosmetic surface.
Warp
Design a part with walls too thin for the target material and it’s likely to curl up like a potato chip. This is called warp, and is easily avoided by following the same rules used with sink, namely staying within the general wall thickness guidelines. Ironically, the glass-filled materials that work well with sink-prone parts are more susceptible to warp. That’s because, as a part cools, the glass fibers tend to line up in the same direction, creating internal stresses. Parts with internal support structures — gussets to support thin walls, or ribbing of large flat surfaces — fare best against warp.
Flash
With free flowing materials such as Santoprene or unfilled nylon, a small amount of flash can sometimes ooze into the seam, and often requires trimming once the part has cooled. Many orthogonal parts have sharp corners, which make a clean, crisp junction at which the mold can separate. Flash or no, you should expect a parting line on most molded products, but there are ways of modifying the part geometry to avoid one.
Knit Lines
Worried about those fine lines that look like hairline cracks in your injection-molded part? Don’t be. Those are knit lines, formed when two opposing flows of material join together in the mold cavity. Commonly seen at the edge of a hole or other cored feature, knit lines are — as a rule — purely cosmetic, but may create a physical failure point if present in an area of the part that receives substantial stress, such as the head of a screw. In this case, designing a strengthening boss feature around the hole is a good precaution, or just skip the hole entirely and drill it afterwards.
Drag
Sufficient draft is an important part of any mold design, and quick-turn tooling is no exception. Vertical walls, meaning those part surfaces parallel to the direction of mold operation, should have a minimum draft angle of 1/2 degree, and 2 degrees is even better; heavily textured surfaces may require 5 degrees or more. Without proper draft, part ejection becomes difficult if not impossible, and drag or scrape lines may occur.
Vestiges
Gate vestige is that small spot at one end of the part left by removal of the gate after molding, usually with a side cutter or razor knife. It’s an unavoidable fact of injection molding. The only thing, for the most part, that can be done to avoid it is orienting the part in the mold such that cosmetic surfaces are unaffected. There may be options to change a gate style depending on the material and part geometry. It is much easier to do this during the review stages rather than after the mold design stages have begun.
Jets, Orange Peels, Etc.
There are several miscellaneous problems that can crop up with injection molding, several of which can be tied back to wall thicknesses that exceed general recommendations:
- Jetting, a wormlike swirl that appears near especially thick gate areas, is caused by temperature variations within the material flow.
- Similarly, a surface that looks like an orange peel can be caused by flow variations in the mold cavity, usually in thicker sections of the part.
- Silvery streaks and material flaking is known as splay, and can occur as a result of moist or degraded resin, but can also be caused by material shear due to higher than normal injector-screw speeds.
- Blush, a cloudy discoloration normally found near gate areas, can be caused by improper fill speeds, but proper part geometry and gate placement also play a factor.
Most of these issues can be resolved through slight modifications to part design and/or selecting a different material. Difficult part geometries often require fine-tuning of the molding temperature, injection speed, hold times, or all three. Material selection also plays a big part with cosmetics. Two examples of this are polypropylene and HDPE, which tend to sink more than polybutlylene or acetal, but flow better into small part details. It is possible to test different materials using the same mold, but unfortunately shrink factors may prevent parts from having dimensions that match the CAD. In some cases after testing multiple materials, a new mold may be required for further testing or production parts.




