As modern medical devices push the limits of technology, parylene coatings are finding more places to assist evolving technologies. Parylene is a conformal polymer coating that is biostable and biocompatible. It is suited to a multitude of industries and applications due to its ability to be implanted, outstanding barrier protection, electrical insulation properties, and unique deposition method.
The coating process
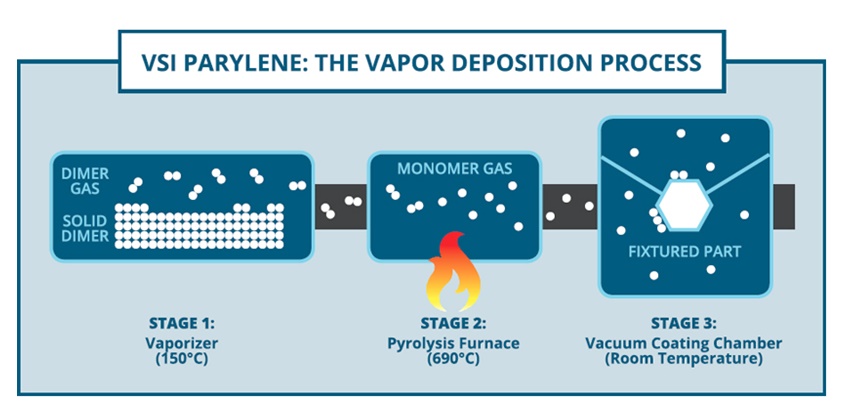
Parylene is applied in a three stage, vapor-deposition process. It lets the material deposit molecule by molecule onto parts placed in a vacuum chamber. This creates an extremely conformal coating that evenly covers grooves, crevices, gaps, and even sharp points. Because the coating is applied molecule by molecule the thickness can be controlled to the micron.
The process works this way:
Stage 1: Parts are fixtured into a vacuum coating chamber. The solid parylene dimer – in powder form – is placed inside the vaporizer where the material turns into a dimer gas.
Stage 2: The dimer gas then flows into the pyrolysis furnace which heats the dimer gas and turns it into a monomer (single molecule) gas.
Stage 3: Finally, the monomer gas enters a room-temperature deposition chamber, which contains the fixtured parts, where the parylene deposits itself molecule-by-molecule onto everything in the chamber to create a thin and highly conformal coating.
Other considerations
While the parylene process has several advantages over dip and spray coatings, there are process considerations. For example, the adhesion of parylene to the substrate is critically important in every application. To get the best adhesion, it is important that parts are clean and free of oils and debris. With a clean substrate, additional measures such as liquid surface activators or plasma treatment can be used to improve the bond between parylene and the substrate.
Because parylene evenly deposits on every surface in the vacuum chamber, areas that must remain free of coating are masked, or the coating is removed afterwards. When coating removal is necessary, it is typically done with a laser or plasma ablation. During the device and component design stage, identifying the areas that must remain coating free can greatly improve downstream processing efficiency.
Inspection and testing
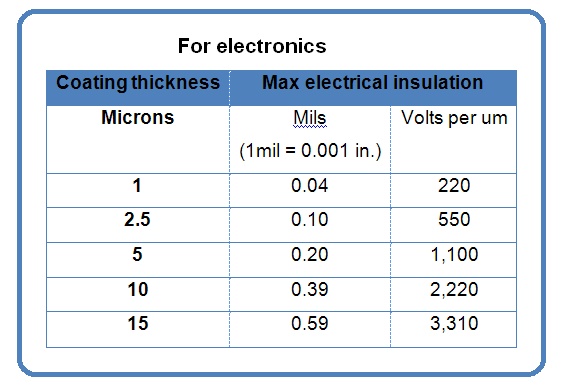
Quality attributes for parylene typically specify the coating thickness, area of coverage, visual, and adhesion-testing requirements. Thin films can be measured non-destructively using spectral reflectance directly on the parts or by measuring witness coupons that were coated with the parts.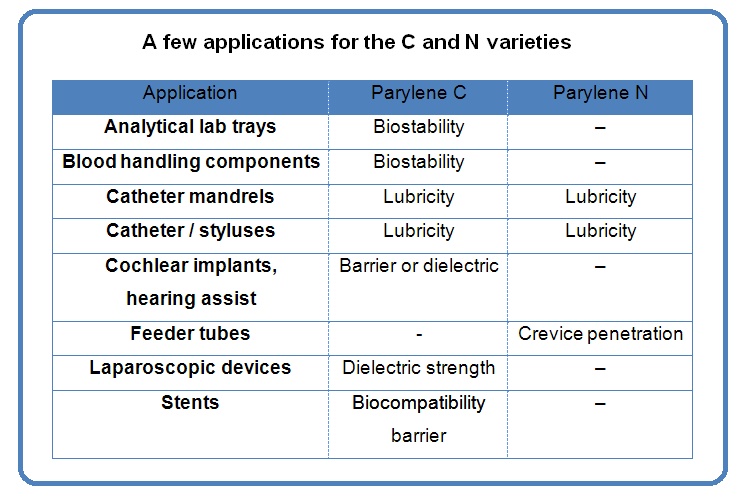
Important properties
Parylene typically becomes the material of choice when an ultra-thin, pinhole free coating is required for implantation, a dielectric barrier, a chemical and moisture barrier, or dry-film lubricity.
The two most commonly used variants of parylene are type “C” and type “N”. Depending on the application, the variant selected can optimize deposition time, crevice penetration, lubricity, dielectric strength, and barrier permeability properties.
Both parylene types are FDA approved and have a USP XXII, Class VI bio-compatibility rating, which make them a perfect fit for medical device applications.
Because each parylene coating application is unique, it is helpful to develop the coating process along with the medical device to help ensure the coating process is scaleable and meets the required quality requirements. Process design conversations will include discussions around selecting the proper thickness, selecting the proper parylene type, allowable clearances, dielectric protection requirements, barrier protection requirements, and cost expectations. Working with an experienced and innovative parylene coating service provider will ensure the best solution is found. Lead time is also critical when selecting a parylene coating provider because faster iterations help a design evolve quicker and reduce time to market.